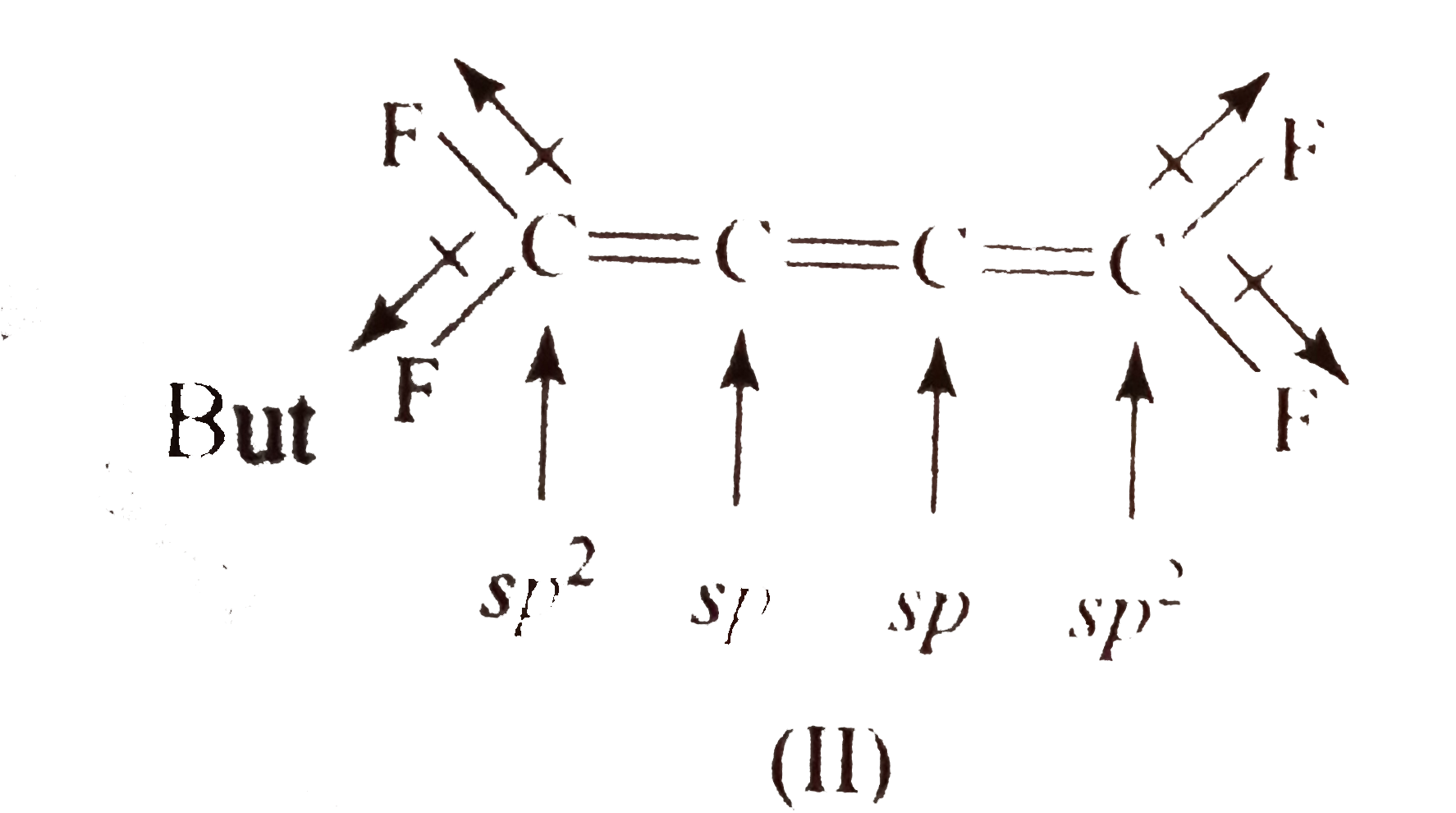A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ALKENES AND ALKADIENES
CENGAGE CHEMISTRY|Exercise Multiple Correct Answer Type|41 VideosALKENES AND ALKADIENES
CENGAGE CHEMISTRY|Exercise Single Correct Answer Type|62 VideosALKENES AND ALKADIENES
CENGAGE CHEMISTRY|Exercise EXERCISES|29 VideosALKANES AND CYCLOALKANES
CENGAGE CHEMISTRY|Exercise Archives|13 VideosALKYNES
CENGAGE CHEMISTRY|Exercise Exercises (Archives - Analytical and Desriptive Type)|4 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-ALKENES AND ALKADIENES-linked Comprehension Type
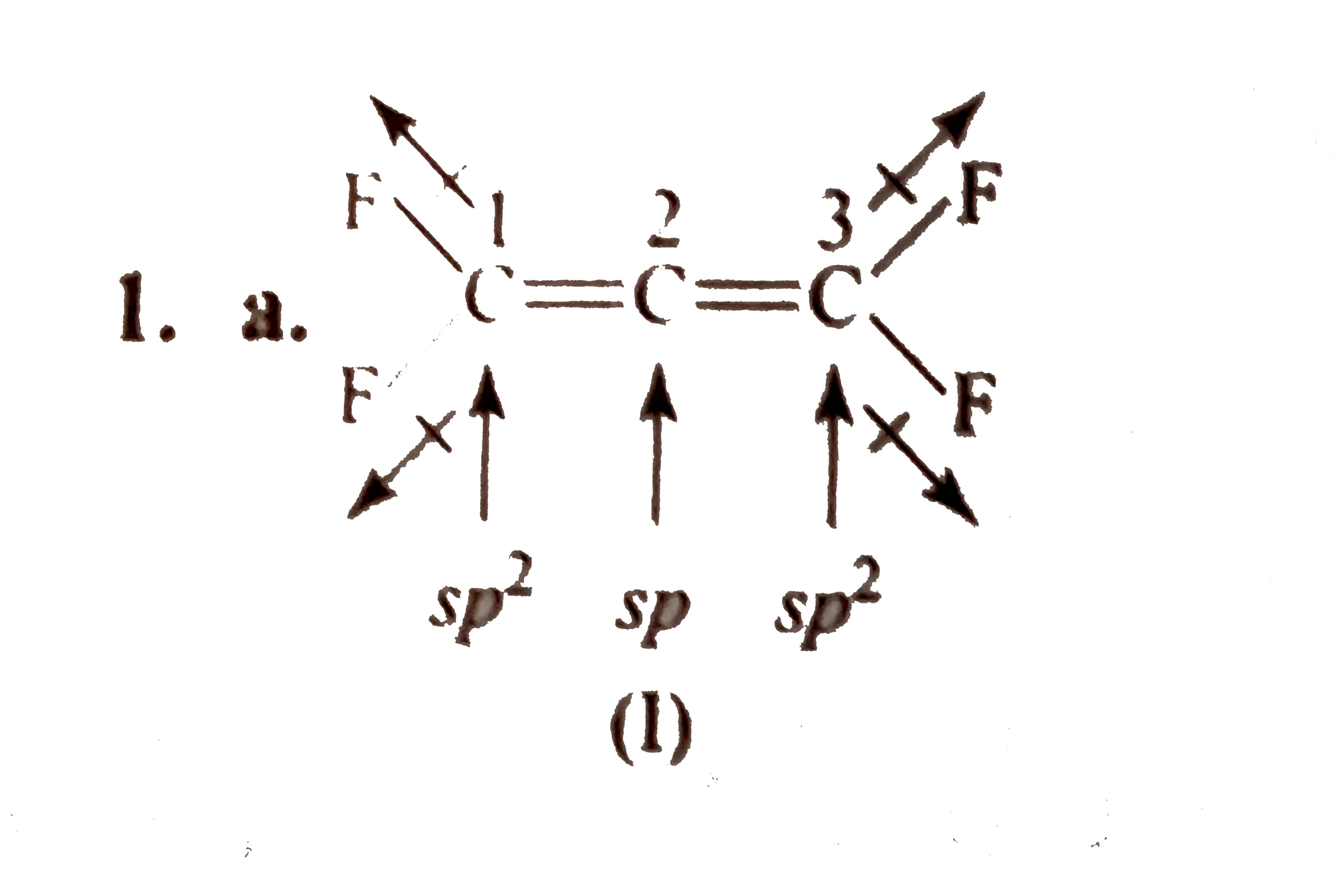
- The structures to two compound (I) and (II) are given below. Which sta...
Text Solution
|
- The structures to two compound (I) and (II) are given below. Which sta...
Text Solution
|
- The structures to two compound (I) and (II) are given below. Which sta...
Text Solution
|
- A naturally occuring antibiotic called mycomycin 9A) has the structure...
Text Solution
|
- A naturally occuring antibiotic called mycomycin (A) has the structure...
Text Solution
|
- A naturally occuring antibiotic called mycomycin 9A) has the structure...
Text Solution
|
- Compound (A)(C(13)H(24)O) is a sex - attractant pheromone two molar eq...
Text Solution
|
- Compound (A)(C(13)H(24)O) is a sex - attractant pheromone two molar eq...
Text Solution
|
- Compound (B) and (D) are, respectively :
Text Solution
|
- Compound (C) and (E) are, rescpectively:
Text Solution
|
- The formation of (B) and (D) takes place, respectively, when :
Text Solution
|
- The formation of (C) and (E) takes place, respectivley, when :
Text Solution
|
- Compound (A) is :
Text Solution
|
- Compound (B) is :
Text Solution
|
- Compound (C) is :
Text Solution
|
- Reagent (A) is :
Text Solution
|
- Which of the following connot be used as reagent (B) ?
Text Solution
|
- Reagent (C) is :
Text Solution
|
- (A) is :
Text Solution
|
- (B) and (C) , respectively, are :
Text Solution
|