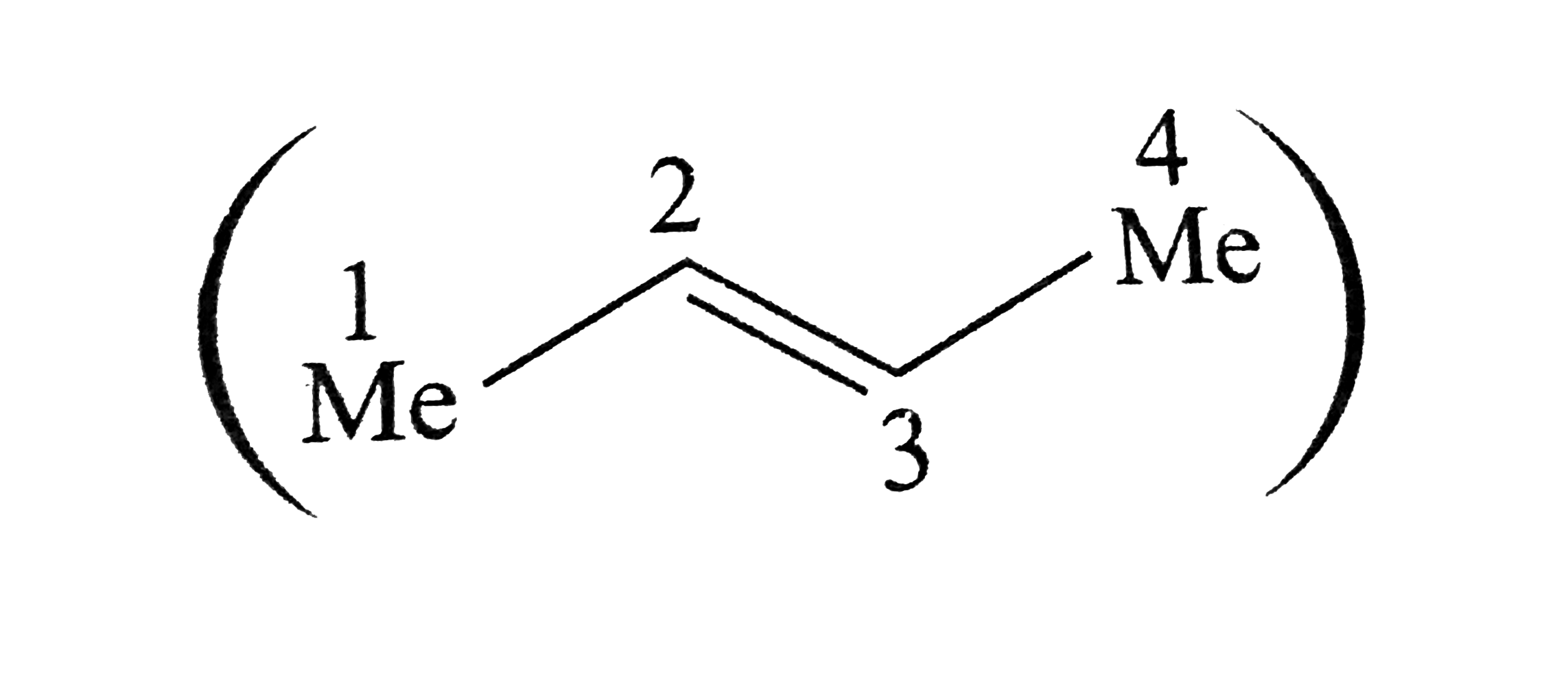
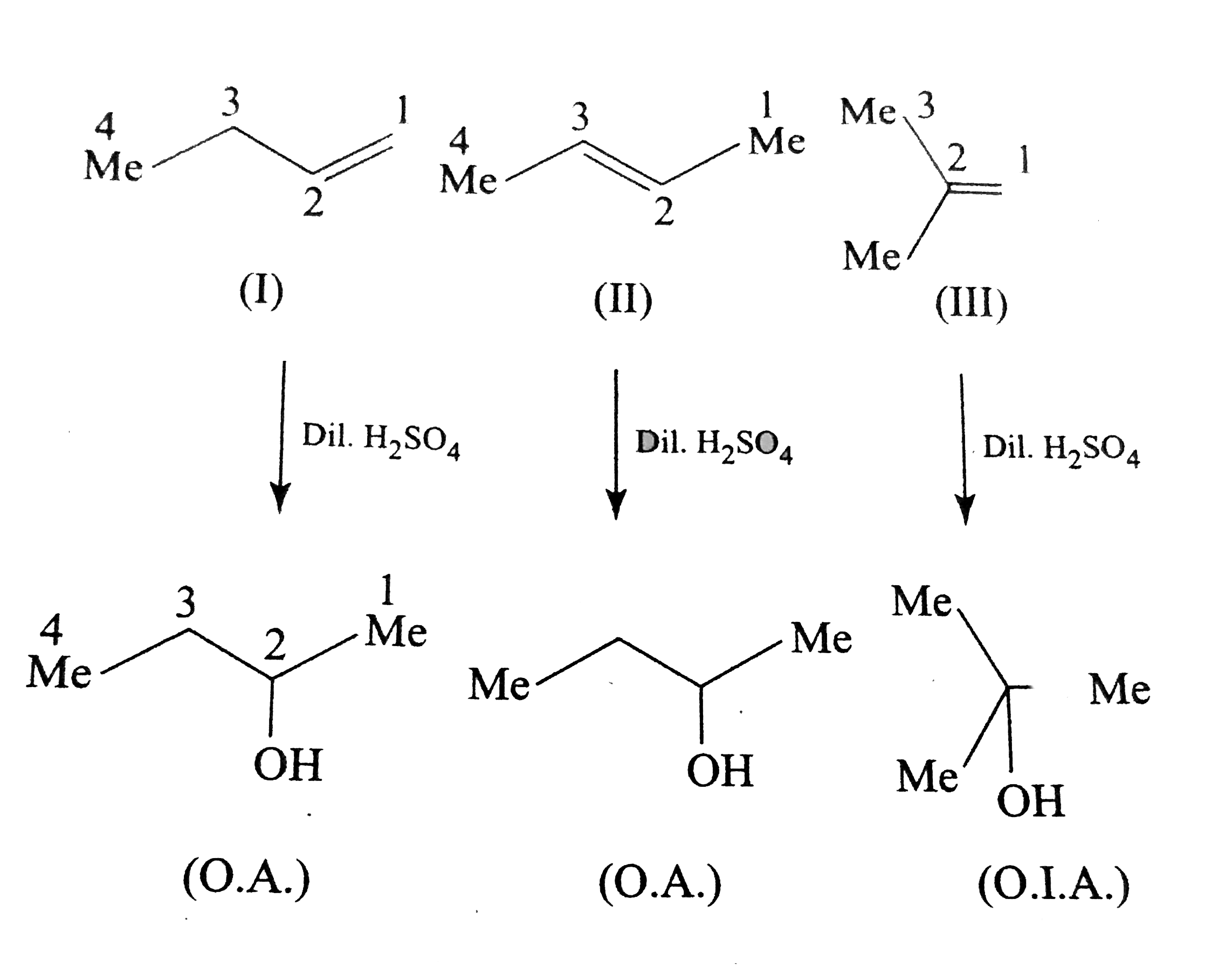
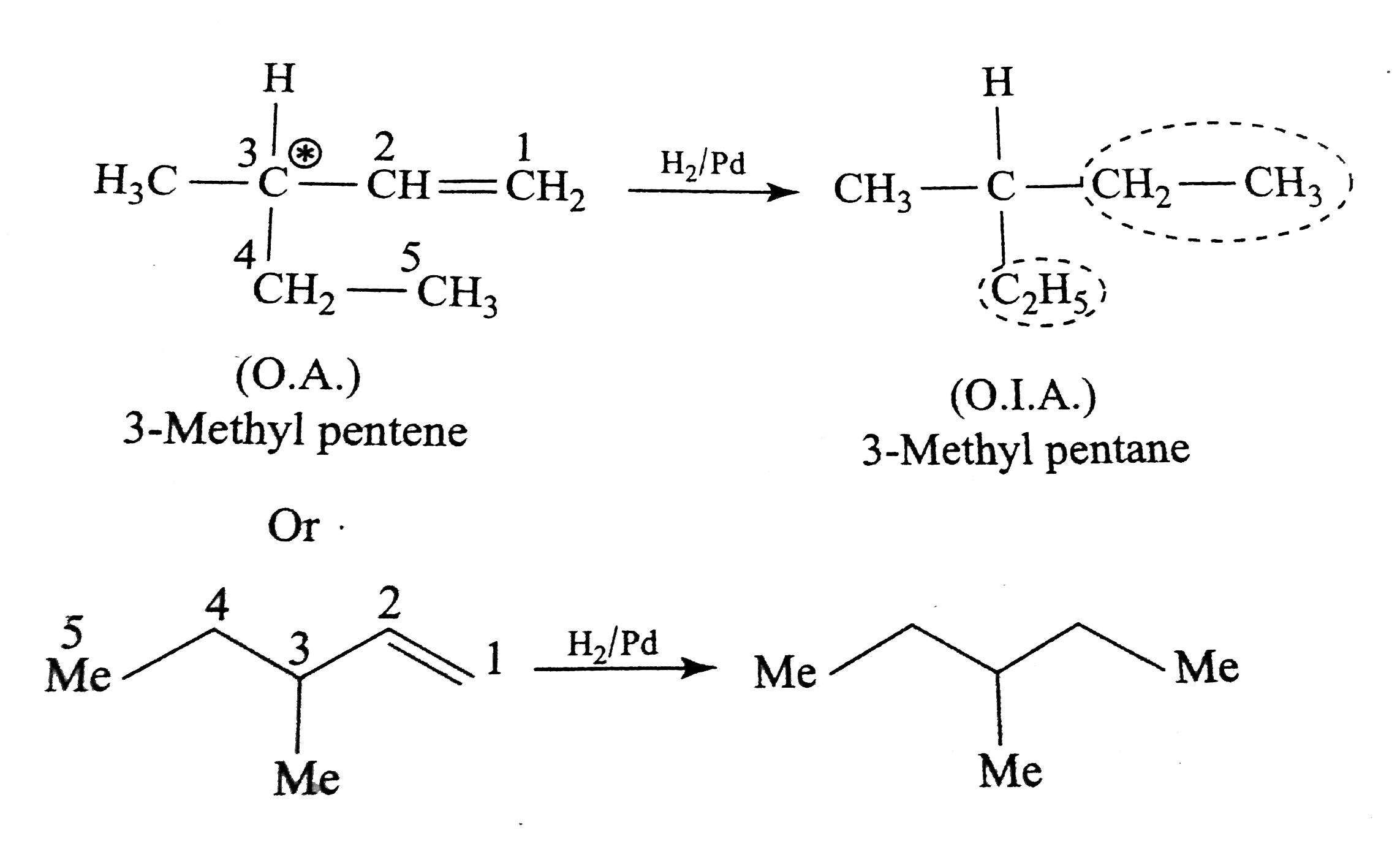
Text Solution
Verified by Experts
Topper's Solved these Questions
ALKENES AND ALKADIENES
CENGAGE CHEMISTRY|Exercise Single correct Answer|14 VideosALKENES AND ALKADIENES
CENGAGE CHEMISTRY|Exercise True/False|1 VideosALKANES AND CYCLOALKANES
CENGAGE CHEMISTRY|Exercise Archives|13 VideosALKYNES
CENGAGE CHEMISTRY|Exercise Exercises (Archives - Analytical and Desriptive Type)|4 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-ALKENES AND ALKADIENES-SUBJECTIVE TYPE
- Write the structural formula of the major product in the following cas...
Text Solution
|
- One mole of a hydrocarbon 'A' reacts with 1 mil of brominne giving a ...
Text Solution
|
- State with balanced equatio, what happens when 'propene is bubbled thr...
Text Solution
|
- Give reasons for the following in one or two sentences: i. Methane d...
Text Solution
|
- The following statement is true only under some specific conditions. W...
Text Solution
|
- A white precipitate was formed slowly when silver nitrate was added to...
Text Solution
|
- Give a chemical test and the reagents used to distinguish between cycl...
Text Solution
|
- Write the balanced chemical equatio for the following : ' Ethylene gly...
Text Solution
|
- Give the structures of (A),(B) and (C)(explanation is not required ). ...
Text Solution
|
- When gas A is passed through dry KOH at low temperature, a deep red co...
Text Solution
|
- An organic compound (E)(C(5)H(8)), on hydrogenation gives a compound ...
Text Solution
|
- Give the structure of the major organic products obtained from 3-ethyl...
Text Solution
|
- An alkyl halide (X)of the folmula C(6)H(13)Cl on treatment with potass...
Text Solution
|
- Complete the foloowing giving the structurs of the principal organic p...
Text Solution
|
- Give reasons for the following in one or two sentences ' The central c...
Text Solution
|
- What would be the major product in the following reaction ?
Text Solution
|
- Monomer (A) of a polymer on ozonolysis yields 2 mil HCHOand 1 mol CH(3...
Text Solution
|
- What is the total number of cyclic structureal as well as stereoisomer...
Text Solution
|