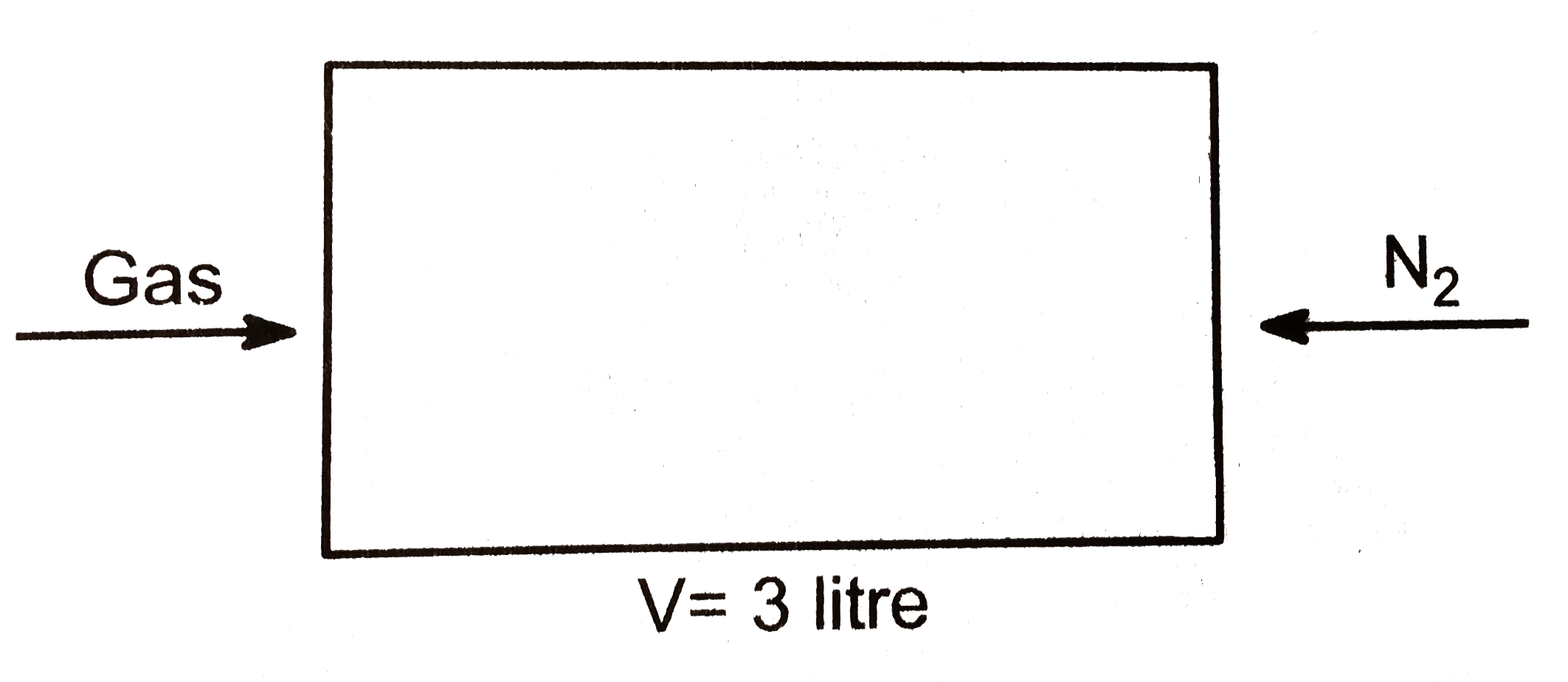
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
P BAHADUR-GASEOUS STATE-Exercise
- Equal volumes of two gases A and B diffuse through a porous pot in 20 ...
Text Solution
|
- The pressure in a bulb dropped from 2000 to 1500 mm Hg in 47 min when ...
Text Solution
|
- For 10 minutes each at 27^(@)C from two identical holes nitrogen and a...
Text Solution
|
- Through the two ends of a glass tube of length 200cm hydrogen chloride...
Text Solution
|
- A mixture of 0.5 mole of CO and 0.5 mole of CO(2) is taken in a vessel...
Text Solution
|
- The pressure in a bulb dropped from 2000 to 1500 mm Hg in 47 min when ...
Text Solution
|
- Pay load is defined as the difference between the mass of displaced ai...
Text Solution
|
- Calculate the total and average kinetic energy of 32 g methane molecu...
Text Solution
|
- Calculate the root mean square speed total and average translational k...
Text Solution
|
- Suppose a gas sample in all have 6xx10^(23) molecules Each 1//3rd of t...
Text Solution
|
- The root mean square speed of gas molecules at a temperature 27K and p...
Text Solution
|
- Calculate root mean square speed most probable speed and average speed...
Text Solution
|
- The average veloctiy of an ideal gas molecule at 27^(@)C is 0.3 m s^(-...
Text Solution
|
- 6.0g he and 12.0 g Ne molecules both having average velocity 4 xx 10^(...
Text Solution
|
- Calculate the compressibility factor for SO(2) if 1 mole of it occupie...
Text Solution
|
- Calculate the compressibility factor for SO(2) if 1 mole of it occupie...
Text Solution
|
- The compressibility factor for a given real gas is 0.927 at 273 K and ...
Text Solution
|
- Comperessibility factor (Z) for N(2) at -50^(@) C and 800 atm pressure...
Text Solution
|
- A real gas is supposed to obey the gas equation P(V-b) =nRT at STP if ...
Text Solution
|
- Calculate the pressure excerted by 5 mol of CO(2) in 1 L vessel at 47^...
Text Solution
|