Text Solution
Verified by Experts
P BAHADUR-GASEOUS STATE-Exercise
- One way of writing the equation of state for real gases is PV=RT[1+(...
Text Solution
|
- The compressibility factor for definite amount of van der Waals' gas a...
Text Solution
|
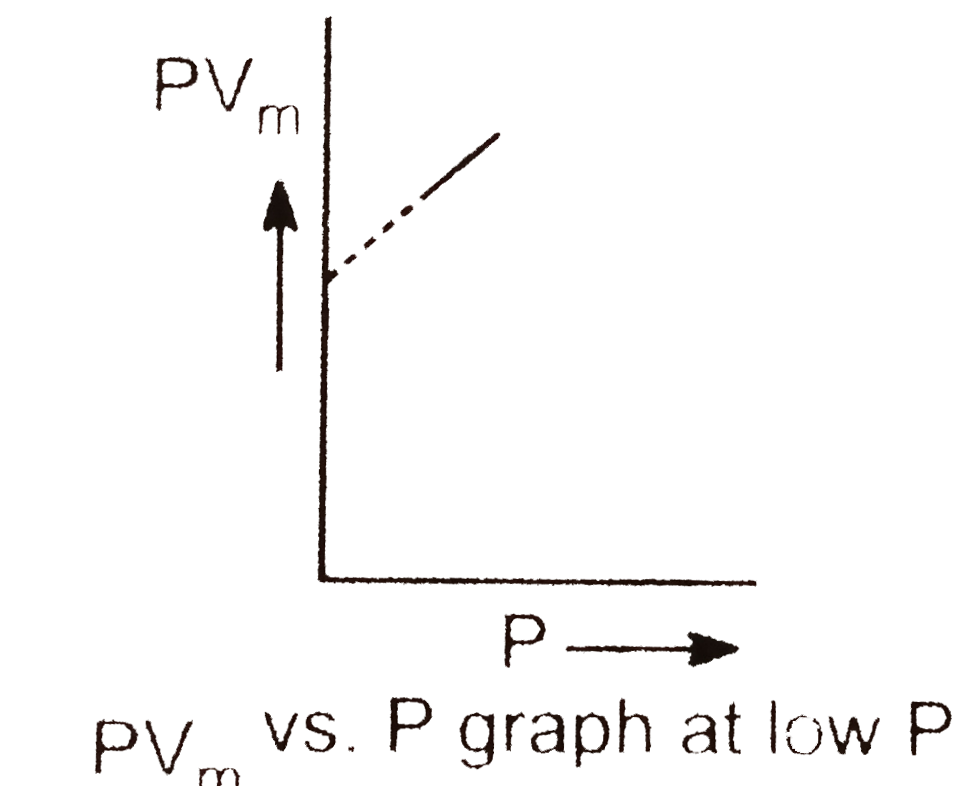
- A graph is plotted between PV(m) along Y-axis and P along X-axis whre ...
Text Solution
|
- A gas can be liquefied most suitably at .
Text Solution
|
- Average velocity of the molecules of a gas in a container moving in on...
Text Solution
|
- An open vessel containing air is heated form 300 K to 400 K. The fract...
Text Solution
|
- I, II, and III are three istherms, respectively, at T(1), T(2), and T(...
Text Solution
|
- NH(3) gas is liquefied more easily than N(2). Hence
Text Solution
|
- Under critical conditions, the compressibility factor foa a gas is .
Text Solution
|
- The behaviour of a real gas is usually depicted by plotting compressib...
Text Solution
|
- When an ideal gas undergoes unrestrained expansion, no cooling occurs ...
Text Solution
|
- For two gases A and B with molecular weights M(A) and M(B), respective...
Text Solution
|
- The temperature at which the second virial coefficient of a real gas i...
Text Solution
|
- The Joule -Thomson coefficient for a gas is zero at .
Text Solution
|
- The team that accounts for intermolecular force in van der Waals' equa...
Text Solution
|
- Total number of electrons in 1.4g dinitrogen gas is .
Text Solution
|
- Consider 1 cm^(3) sample of air at absolute temperature T(0) at sea le...
Text Solution
|
- Boyle's law may be experssed as .
Text Solution
|
- According to Charles's law
Text Solution
|
- For an ideal gas, the correct relation is .
Text Solution
|
 .
.