A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
P BAHADUR-GASEOUS STATE-Exercise
- If one mole of a monoatomic gas (gamma=7//53) is mixed with one mole o...
Text Solution
|
- If the pressure of N(2)//H(2) mixture in a closed vessel is 100 atmosp...
Text Solution
|
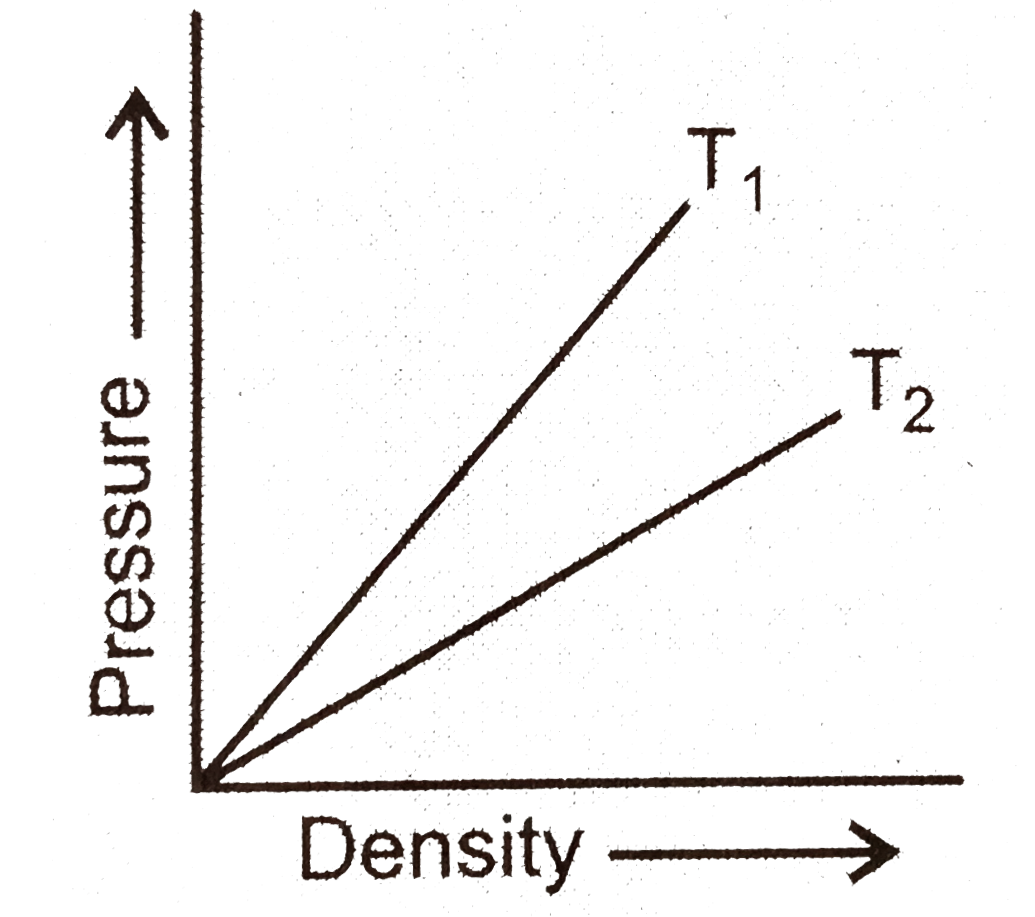
- Figure shows graphs of pressure versus density for an ideal gas at two...
Text Solution
|
- The ratio a/b (the terms u8sed in van der Waals' equation) has the uni...
Text Solution
|
- A gas in an open container is heated from 27^(@)C to 127^(@)C The frac...
Text Solution
|
- The circulation of blood in human body supplies O(2) and releases CO(2...
Text Solution
|
- The van der Waals equation is [P+(n^(2)a)/(V)] (V -nb) =nRT for n mole...
Text Solution
|
- A V dm^(3) flask contains gas A and another flask of 2V dm^(3) contain...
Text Solution
|
- Consider an ideal gas contained in a vessel If the intermolecular inte...
Text Solution
|
- The critical temperature of O(2) and N(2) are 155K and 126 K respecti...
Text Solution
|
- At a constant temperature what should be the percentage increase in pr...
Text Solution
|
- At a constant temperature what should be the percentage increase in pr...
Text Solution
|
- When an ideal diatomic gas is heated at constant pressure the fraction...
Text Solution
|
- One mole of an ideal monoatomic gas is mixed with 1 mole of an ideal d...
Text Solution
|
- At low pressure the van der Waals' equation is reduced to [P +(a)/(V^(...
Text Solution
|
- The correct order of temperatures for a real gas is Boyle temp, Critic...
Text Solution
|
- Which pair of gases will show same rate of diffusion at same pressure ...
Text Solution
|
- Calculate the pressure exerted by one mole of CO(2) gas at 273 K van d...
Text Solution
|
- Equal moles of CO,B(2)H(6),H(2) and CH(4) are placed in a container If...
Text Solution
|
- Which of the following statement is incorrect ? .
Text Solution
|
 .
.