A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
P BAHADUR-GASEOUS STATE-Exercise
- A jar contains He and H(2) in the molar ratio 1:5 The ration of mean t...
Text Solution
|
- Which of the following statement about Maxwell Boltzmann law of distri...
Text Solution
|
- The Maxwell-Boltzmann distribution law of molecular speeds is graphica...
Text Solution
|
- Consider the following statements PV(m)=RT(1+(\B)/(V(m))+(B)/(V(m)^(2)...
Text Solution
|
- Which of the following statements on critical constants of gases are c...
Text Solution
|
- Consider the following statements If th van der Waals' parameters of t...
Text Solution
|
- At low pressure the van der Waals' equation is written as .
Text Solution
|
- A 5 litre flask containing 1.0g of hydrogen is heated from 300 K to 60...
Text Solution
|
- At very high pressure, the van der Waals equation reduces to
Text Solution
|
- Which of the following are the characteristics of real gas ? .
Text Solution
|
- At constnat volume for a fixed number of mole of a gas the pressure of...
Text Solution
|
- Volume coefficient at 273 K according to Charles' law is .
Text Solution
|
- A gas obeys P(V-b) =RT Select the correct statement about this gas .
Text Solution
|
- Which of the following statements are incorrect .
Text Solution
|
- If m(1),m(2) are masses of an ideal gas, then which of the graph reper...
Text Solution
|
- A gas described by van der Waals equation .
Text Solution
|
- Two vassels having equal volumes contains H(2) and He at 1 and 2 atm r...
Text Solution
|
- Select the correct statements (s).
Text Solution
|
- Select the correct statements (s).
Text Solution
|
- As the pressure approaching zero at very low pressure the curves plott...
Text Solution
|
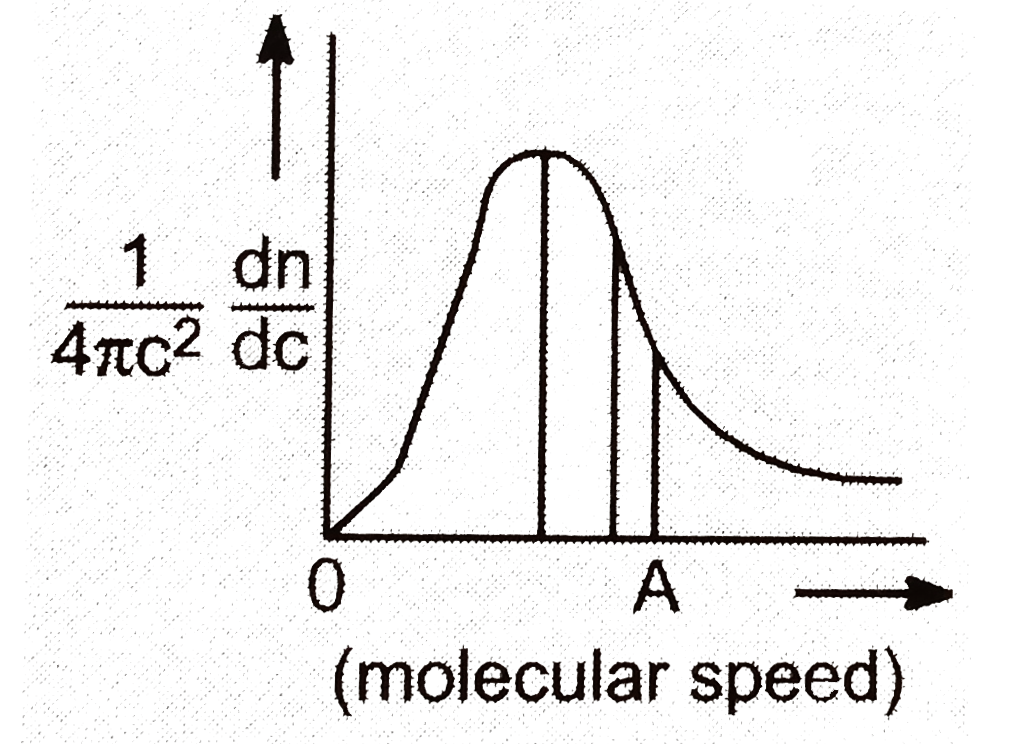 The curve has which of the following characteristics ?.
The curve has which of the following characteristics ?.