A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
P BAHADUR-GASEOUS STATE-Exercise
- Select the correct statements (s).
Text Solution
|
- Select the correct statements (s).
Text Solution
|
- As the pressure approaching zero at very low pressure the curves plott...
Text Solution
|
- Kinetic theory of gases proves .
Text Solution
|
- For an ideal gas number of moles per litre in terms of its pressure P ...
Text Solution
|
- According to kinetic theory of gases in an ideal gas between two succ...
Text Solution
|
- As the temperature is raised from 20^(@)C to 40^(@)C the averge kineti...
Text Solution
|
- In van der Waals' equation of state of the gas law the constnat 'b' is...
Text Solution
|
- Which one of the following statement is not true about the effect of a...
Text Solution
|
- If 10^(-4) dm^(3) of water is introduced into a 1.0 dm^(3) flask to 30...
Text Solution
|
- a' and 'b' are van der Waals' constants for gases Chlorine is more eas...
Text Solution
|
- The compressibility factor for a real gas at high pressure is .
Text Solution
|
- Arrange the van der Waals constant for the gases .
Text Solution
|
- XmL of H(2) gas effuses through a hole in a container is 5 second. The...
Text Solution
|
- The compressibility factor for an ideal gas is .
Text Solution
|
- According to Graham's law, at a given temperature the ration of rates ...
Text Solution
|
- A gas will approach ideal behaviour at
Text Solution
|
- The compressibility of a gas is less than unity at STP .
Text Solution
|
- The rms velocity of hydrogen is sqrt7 times the rms velocity of nitrog...
Text Solution
|
- At 100^(@)C and 1 atm, if the density of the liquid water is 1.0 g cm^...
Text Solution
|
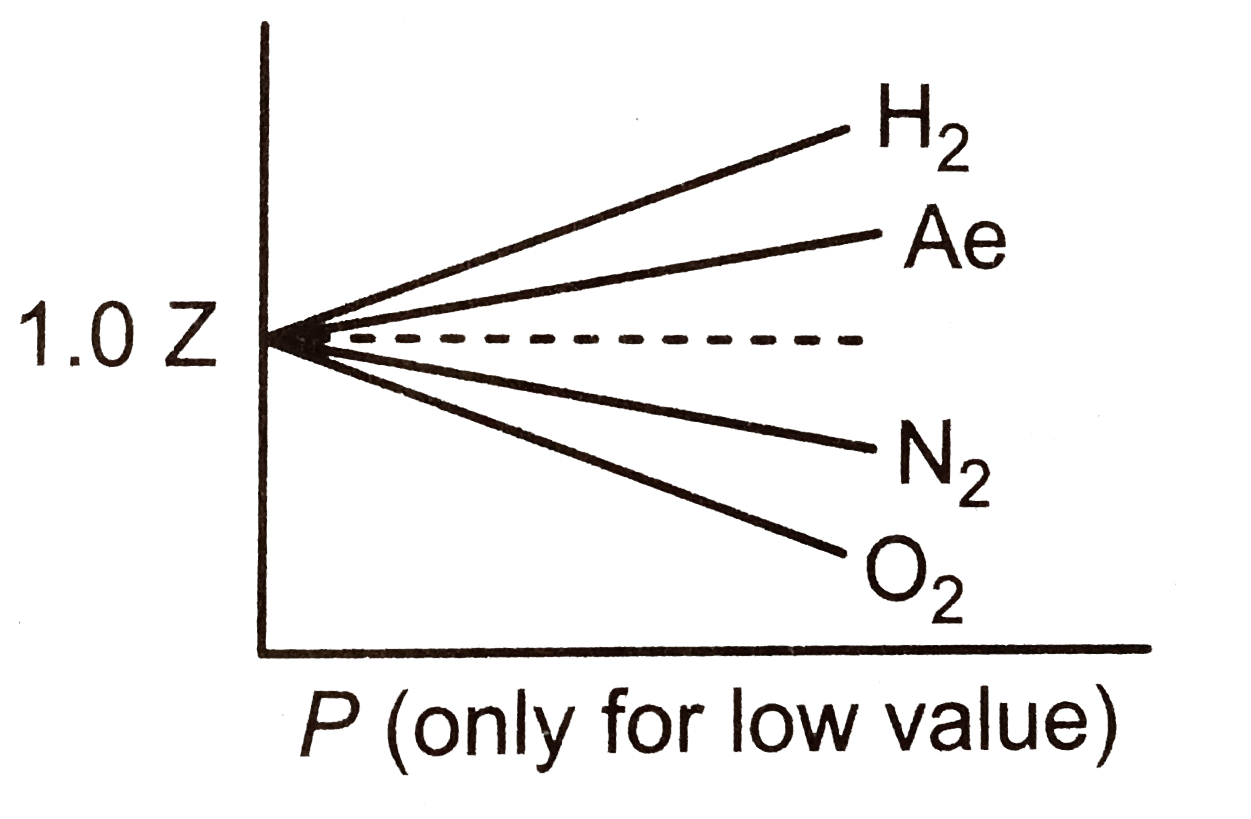 .
.