A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
P BAHADUR-GASEOUS STATE-Exercise
- The given graph represents the variations of compressibility factor Z=...
Text Solution
|
- The term that is correct for the attractive forces present in a real g...
Text Solution
|
- For one mole of a van der Waals gas when b =0 and T =30 K the PV vs1//...
Text Solution
|
- 7g of a gas occupies a volume of 4.1 litre at 300 K If mol wt of gas ...
Text Solution
|
- 4 litre C(2)H(4) (g) burns in oxygen at 27^(@)C and 1 atm to produce C...
Text Solution
|
- A certain amout of an ideal gas occupies a volume of 1.0m^(3) at a giv...
Text Solution
|
- A container having 3 mole of gas occupies 60 litre at pressure P and t...
Text Solution
|
- Density of a gas is found to be 5.46//dm^(3) at 27^(@)C at 2 bar press...
Text Solution
|
- Calculate the volume occupied by 96 g CH(4) at 16 atm and 27^(@)C(R -0...
Text Solution
|
- 1 litre capacity flask containing NH(3) at 1 atm and 25^(@)C A spark i...
Text Solution
|
- A compound exists in the gaseous phase both as monomer (A) and dimer (...
Text Solution
|
- 5.6 litre of an unknown gas at NTP requires 12.5 calorie to raise its ...
Text Solution
|
- Caluculate the ratio of the rate diffusion of He and CH(4) under ident...
Text Solution
|
- At 400K the root mean square (rms) speed of a gas x (mol. wt. =40) is ...
Text Solution
|
- At same temperature two bulbs A and B of equal capactiy are filled wit...
Text Solution
|
- The Graham's law states that ''at constant pressure and temperature th...
Text Solution
|
- An L.P.G cylinder contains 15kg of butane gas at 27^(@)C and 10 atm pr...
Text Solution
|
- Sulphur vapour (Sn) diffuses through a porous plug at 0.354 rate of di...
Text Solution
|
- A cylindrical container with moveable piston initially hold 3.0 mole o...
Text Solution
|
- At identical temperature and pressure the rate of diffusion of hydroge...
Text Solution
|
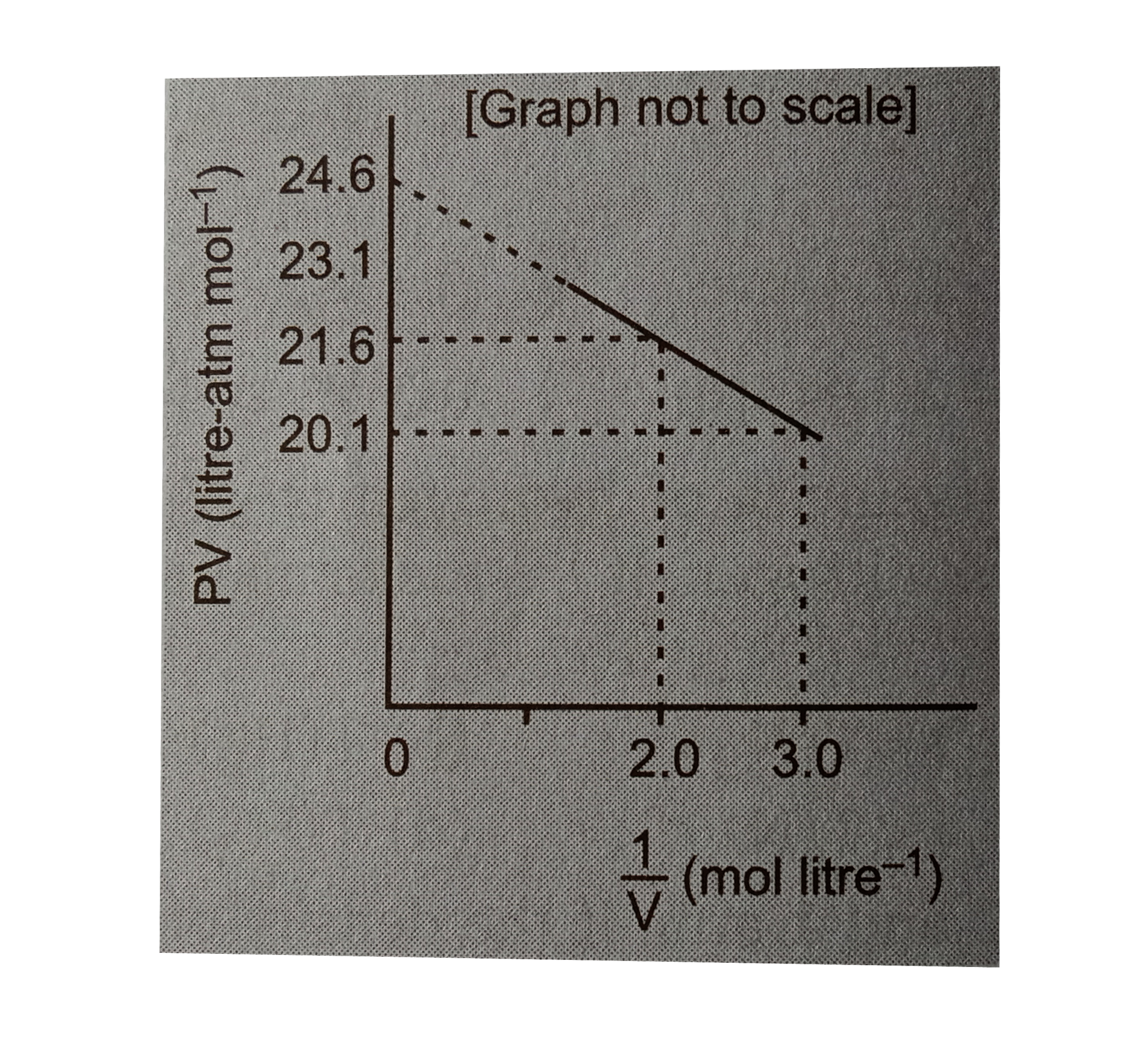 .
.