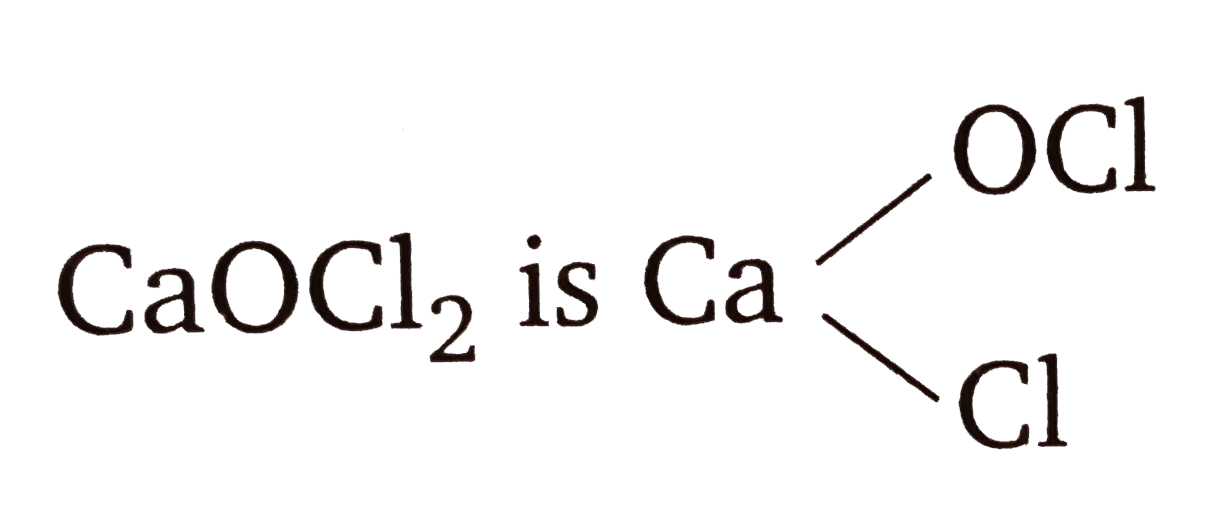A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
REDOX REACTIONS
P BAHADUR|Exercise Exercise 3B Objective problems|18 VideosREDOX REACTIONS
P BAHADUR|Exercise Exercise (4) Objective problems|15 VideosREDOX REACTIONS
P BAHADUR|Exercise Exercise(2) previous year numerical problems|30 VideosRADIO ACTIVITY
P BAHADUR|Exercise Exercies 9|99 VideosTHERMOCHEMISTRY
P BAHADUR|Exercise Exercise 9 Advance numerical problems|31 Videos
Similar Questions
Explore conceptually related problems
P BAHADUR-REDOX REACTIONS-Exercise 3(A) Objective problems
- Oxidation number of S in [(CH(3))(2)SO] is:
Text Solution
|
- The equivalent weight of salt KHC(2)O(4).H(2)C(2)O(4).4H(2)O when us...
Text Solution
|
- The oxidation number of Cl in CaOCl(2) is
Text Solution
|
- Oxidation number of carbon in carbon sub-oxide is:
Text Solution
|
- The colour of K(2)Cr(2)O(7) changes from red-orange to lemon-yellow on...
Text Solution
|
- 50 mL of 0.1M solution of a salt reacted with 25 mL of 0.1M solution o...
Text Solution
|
- In a reaction, 4 mole of electrons are transferred to 1 mole of HNO(3)...
Text Solution
|
- During developing of an exposed camera, film one step involves in the ...
Text Solution
|
- The ratio of equivalent weights of C(2)H(5)OH in the following reactio...
Text Solution
|
- An element A in a compound ABD has oxidation number A^(n-). It is oxi...
Text Solution
|
- N(2) + 3 H(2) rarr 2NH(3) Molecular weight of NH(3) and N(2) are x(1...
Text Solution
|
- Equivalent weight of FeC(2)O(4) during its reaction with KMnO(4) is:
Text Solution
|
- The no.of electrons involved in the change, Fe(3)O(4)rarrFe(2)O(3):
Text Solution
|
- How many gram of I(2) are present in a solution which requires 40 mL o...
Text Solution
|
- What weight of FeSO(4) ( mol.wt. =152) will be oxidised by 200 mL of n...
Text Solution
|
- 25 mL of 0.50M H(2)O(2) solution is added to 50 mL of 0.20 M KMnO(4) i...
Text Solution
|
- What volume of O(2) measured at standard condition will be formed by t...
Text Solution
|
- The number of Fe^(2+) ion oxidised by one mole of MnO(4)^(-) ions is:
Text Solution
|
- What mass of HNO(3) is needed to convert 5g of the iodine into iodic a...
Text Solution
|
- Number of K^(+) ions present in one litre of M//5KMnO(4) solution are:
Text Solution
|