A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Complex : Charge of Co is
Text Solution
|
- Which among the following statements are true for the complex [Co(NH(3...
Text Solution
|
- Complex : Charge of Co is
Text Solution
|
- The charge number , oxidation number and coordination number of the co...
Text Solution
|
- Statement-1: [Co^(II)(gly)(3)] is called inner-metallic complex or inn...
Text Solution
|
- [Ni(CO)4] is a …………complex.
Text Solution
|
- Which among the following statement are true for the complex [Co(NH(3)...
Text Solution
|
- charging and discharging of capacitors in complex circuits
Text Solution
|
- charging and discharging of capacitors in complex circuits
Text Solution
|
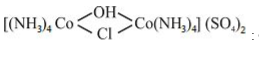 : Charge of Co is
: Charge of Co is