Similar Questions
Explore conceptually related problems
Recommended Questions
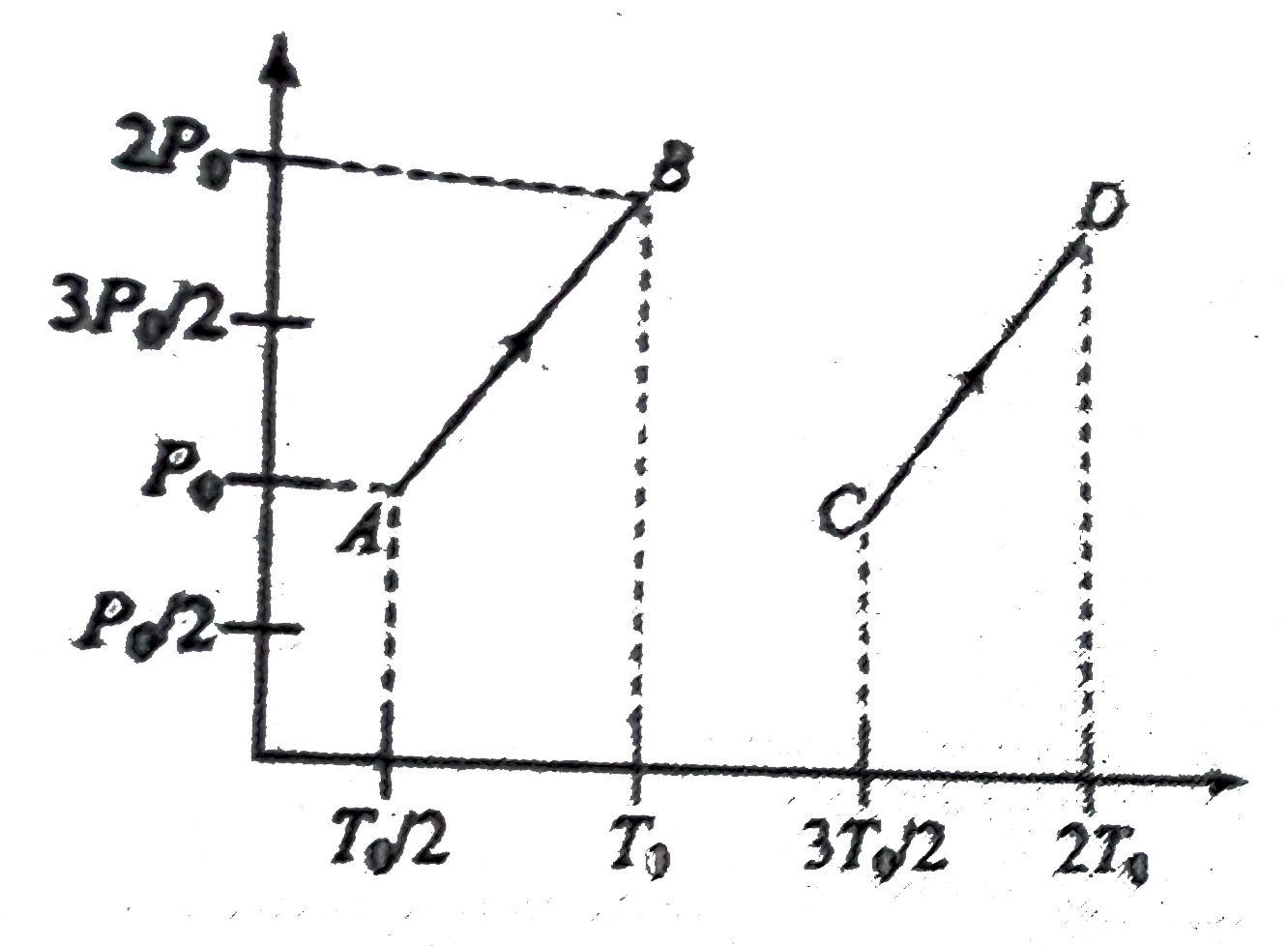
- P-T graph for same number of moles of two ideal gases are shown . Fin...
Text Solution
|
- keeping the number of moles, volume and temperature the same, which of...
Text Solution
|
- The P-T graph for the given mass of an ideal gas is shown in figure. T...
Text Solution
|
- P-T graph for same number of moles of two ideal gases are shown . Fin...
Text Solution
|
- The p-T graph for the given mass of an ideal gas is shown in figure. W...
Text Solution
|
- Two gases A and B at same pressure contain number of moles n(1) and n(...
Text Solution
|
- निम्न में से कौन - सा आयतन (V), ताप (T) ग्राफ ( आलेख ) , एक वायुमंडल...
Text Solution
|
- Pressure temperature (p-1) graph of n moles of an ideal gas is shown i...
Text Solution
|
- मोल आदर्श गैस के व्यवहार को एक वायुमण्डलीय दाब पर प्रदर्शित किया गया ह...
Text Solution
|