Text Solution
Verified by Experts
Topper's Solved these Questions
PERIODIC CLASSIFICATION OF ELEMENTS
TARGET PUBLICATION|Exercise Apply your knowledge|49 VideosPERIODIC CLASSIFICATION OF ELEMENTS
TARGET PUBLICATION|Exercise Chapter Assessment|9 VideosPERIODIC CLASSIFICATION OF ELEMENTS
TARGET PUBLICATION|Exercise Questions based on diagram|16 VideosMETALLURGY
TARGET PUBLICATION|Exercise CHAPTER ASSESSMENT|19 Videos
Similar Questions
Explore conceptually related problems
TARGET PUBLICATION-PERIODIC CLASSIFICATION OF ELEMENTS -Questions based on paragraph
- In the modern periodic table, the elements are arranged in the increas...
Text Solution
|
- In the modern periodic table, the elements are arranged in the increas...
Text Solution
|
- In the modern periodic table, the elements are arranged in the increas...
Text Solution
|
- In the modern periodic table, the elements are arranged in the increas...
Text Solution
|
- In the modern periodic table, the elements are arranged in the increas...
Text Solution
|
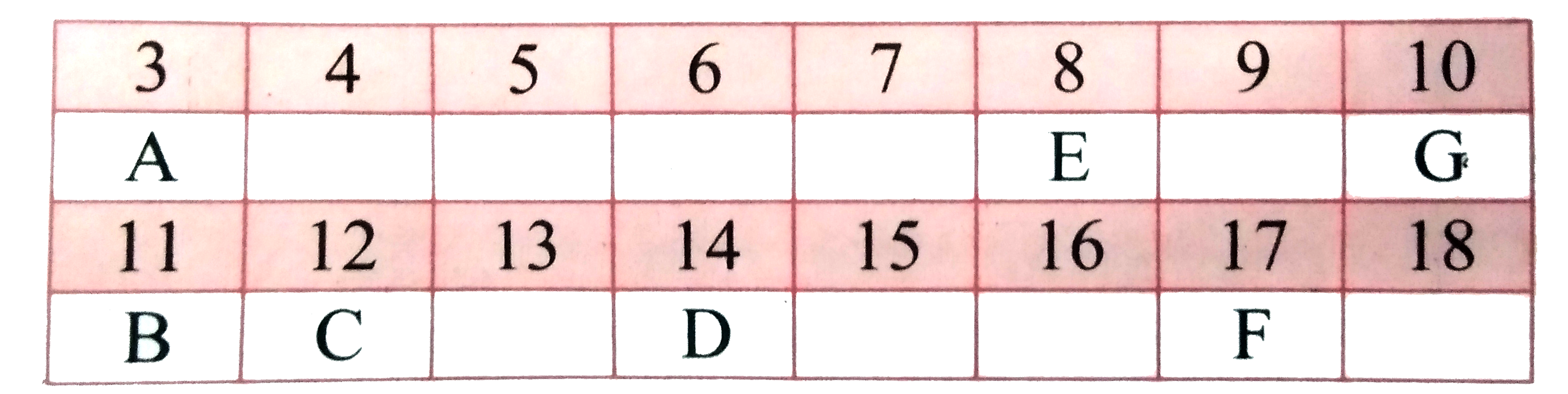
- In the following table, six elements A, B, C, D, E and F (here letters...
Text Solution
|
- In the following table, six elements A, B, C, D, E and F (here letters...
Text Solution
|
- In the following table, six elements A, B, C, D, E and F (here letters...
Text Solution
|
- In the following table, six elements A, B, C, D, E and F (here letters...
Text Solution
|
- In the following table, six elements A, B, C, D, E and F (here letters...
Text Solution
|
- A scientist was studying reactions of metals and nonmetals. He knew gr...
Text Solution
|
- A scientist was studying reactions of metals and nonmetals. He knew gr...
Text Solution
|
- A scientist was studying reactions of metals and nonmetals. He knew gr...
Text Solution
|
- A scientist was studying reactions of metals and nonmetals. He knew gr...
Text Solution
|
- A scientist was studying reactions of metals and nonmetals. He knew gr...
Text Solution
|