Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
TARGET PUBLICATION-PERIODIC CLASSIFICATION OF ELEMENTS -Apply your knowledge
- Would you place the two isotopes of chlorine, Cl-35 and Cl-37 in diffe...
Text Solution
|
- Write the molecular formulae of oxides of the following elements by re...
Text Solution
|
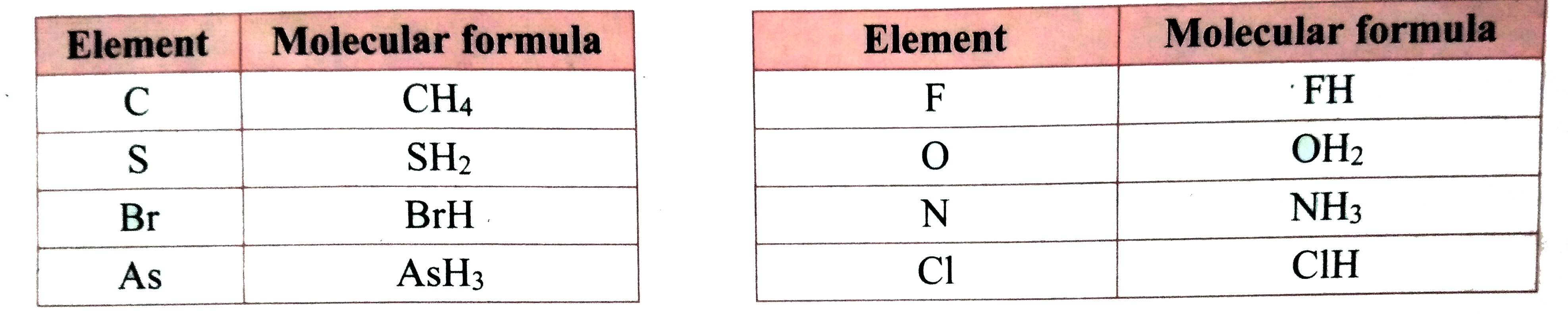
- Write the molecular formulea of compounds of the following elements wi...
Text Solution
|
- Position of the elements in the periodic table: How is the problem...
Text Solution
|
- Position of the elements in the periodic table: How did the positio...
Text Solution
|
- Position of the elements in the periodic table: Can there be an ele...
Text Solution
|
- Position of the elements in the periodic table: What do you think? ...
Text Solution
|
- Go through the modern periodic table (Textbook page no. 23) and write ...
Text Solution
|
- Write the electronic configuration of the first four elements in this ...
Text Solution
|
- Which similarity do you find in their configuration?
Text Solution
|
- How many valence electrons are there in each of these elements?
Text Solution
|
- On going through the modern periodic table, it is seen that the elemen...
Text Solution
|
- Is the number of valence electrons same for all these elements?
Text Solution
|
- Is the number of shells the same in these ?
Text Solution
|
- The elements in the third period, namely, Na, Mg, AI, Si, P, S, CI and...
Text Solution
|
- What are the values of n for the shells L and M?
Text Solution
|
- What is the maximum number of electrons that can be accommodated in a ...
Text Solution
|
- Deduce the maximum electron holding capacity of the shells K, L and M?
Text Solution
|
- What is the relationship between the electronic configuration of an el...
Text Solution
|
- The atomic number of beryllium is 4 while that of oxygen is 8. Write d...
Text Solution
|