Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
TARGET PUBLICATION-PERIODIC CLASSIFICATION OF ELEMENTS -Apply your knowledge
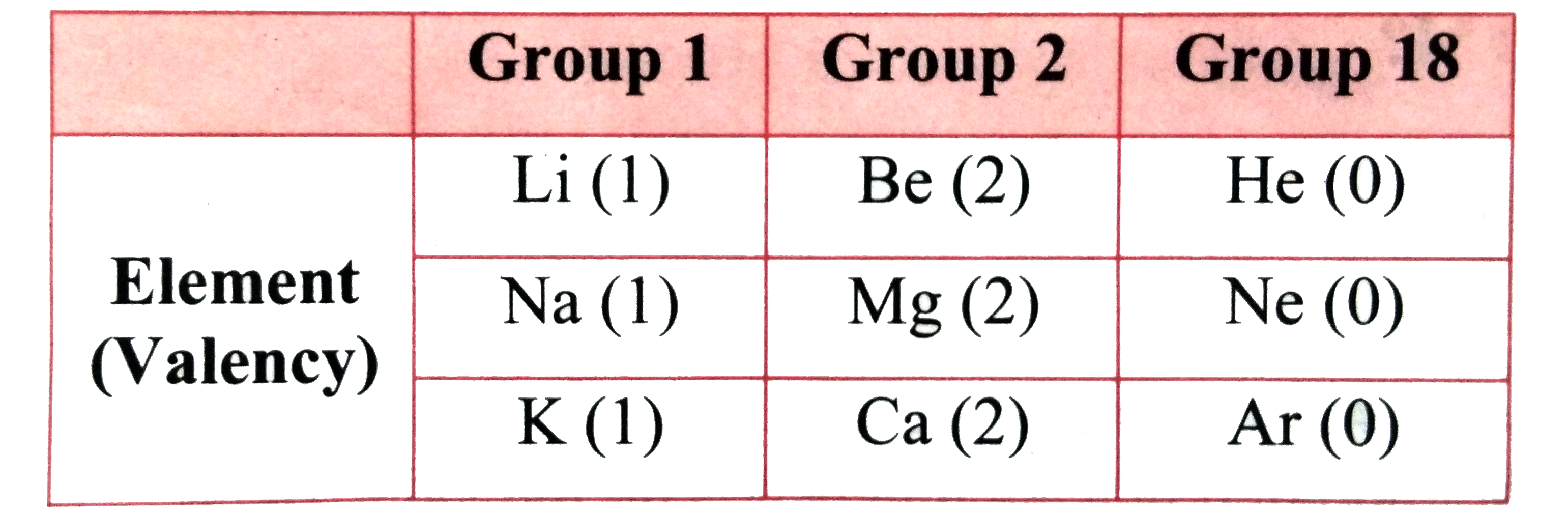
- The table on Textbook page no. 25 is made on the basis of the modern p...
Text Solution
|
- What is the periodic trend in the variation of valency while going fro...
Text Solution
|
- What is the periodic trend in the variation of valency while going dow...
Text Solution
|
- By referring to the modern periodic table, find out the periods to whi...
Text Solution
|
- State the period to which the above elements belong.
Text Solution
|
- Arrange the above elements in a decreasing order of their atomic radii...
Text Solution
|
- Does this arrangement match with the pattern of the second period of t...
Text Solution
|
- Why this arrangement of elements is similar to the above period of the...
Text Solution
|
- Which of the above elements have the biggest and the smallest atom ?
Text Solution
|
- By referring to the modern periodic table, find out the group to which...
Text Solution
|
- Arrange the above elements vertically downwards in an increasing order...
Text Solution
|
- Does this arrangement match with the pattern of the group 1 of the mod...
Text Solution
|
- Which of the above elements have the biggest and the smallest atom ?
Text Solution
|
- What is the periodic trend observed in the variation of atomic radii d...
Text Solution
|
- Look at the elements of third period. Classify them into metals and no...
Text Solution
|
- On which side of the period are the metals ? Left or right?
Text Solution
|
- On which side of the period did you find the nonmetals?
Text Solution
|
- Inert gas elements
Text Solution
|
- Uses of various elements
Text Solution
|
- Find out the applications of all the inert gases, preparea a chart and...
Text Solution
|