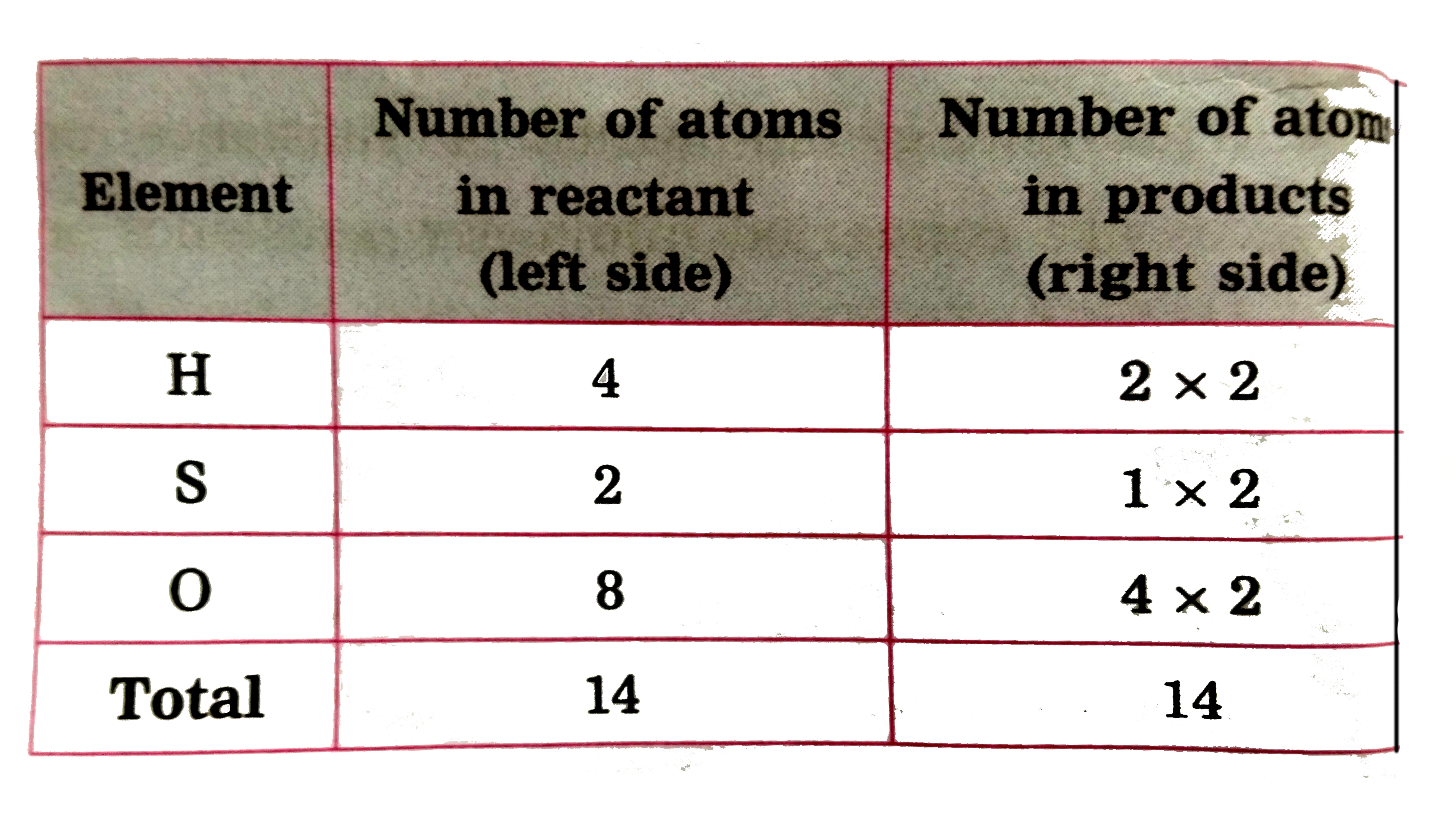
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Write the balanced equations for the following reactions : * (i) H(...
Text Solution
|
- Out of H(2)S(2)O(3),H(2)S(4)O(6),H(2)SO(5) and H(2)S(2)O(8) peroxy aci...
Text Solution
|
- Role of hydrogen peroxide iin the following reaction is respectively. ...
Text Solution
|
- The oxidation number of sulphur in H(2)SO(4),H(2)S(2)O(4) and H(2)S(2)...
Text Solution
|
- Write the balanced equations for the following reactions : (i) H(2)S(2...
Text Solution
|
- Write the balanced equations for the following reactions : * (i) H(2)S...
Text Solution
|
- Write the balanced equations for the following reactions : * (ii) SO(2...
Text Solution
|
- Write the balanced equations for the following reactions : * (iv) H(2)...
Text Solution
|
- Balance the equation stepwise. H(2)S(2)O(7(l))+ H(2)O((l))ot H(2)SO(4(...
Text Solution
|