Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Write the balanced equations for the following reactions : * (ii) SO(2...
Text Solution
|
- For the reaction S(2)O(3)^(2-)(aq) +2H(3)O^(+)(aq) hArr S(s)+H(2)SO(3)...
Text Solution
|
- Predict the outcome of the following reactions and write balanced equa...
Text Solution
|
- Write the balanced equations for the following reactions : (i) H(2)S(2...
Text Solution
|
- निम्नलिखित समीकरणों को पूर्ण कीजिए - (i) Fe(s) + H(2) O(g) to ...
Text Solution
|
- Write the balanced equations for the following reactions : * (i) H(2)S...
Text Solution
|
- Write the balanced equations for the following reactions : * (ii) SO(2...
Text Solution
|
- Write the balanced equations for the following reactions : * (iv) H(2)...
Text Solution
|
- Write the balanced equations for the following reactions : (v) N(2((...
Text Solution
|
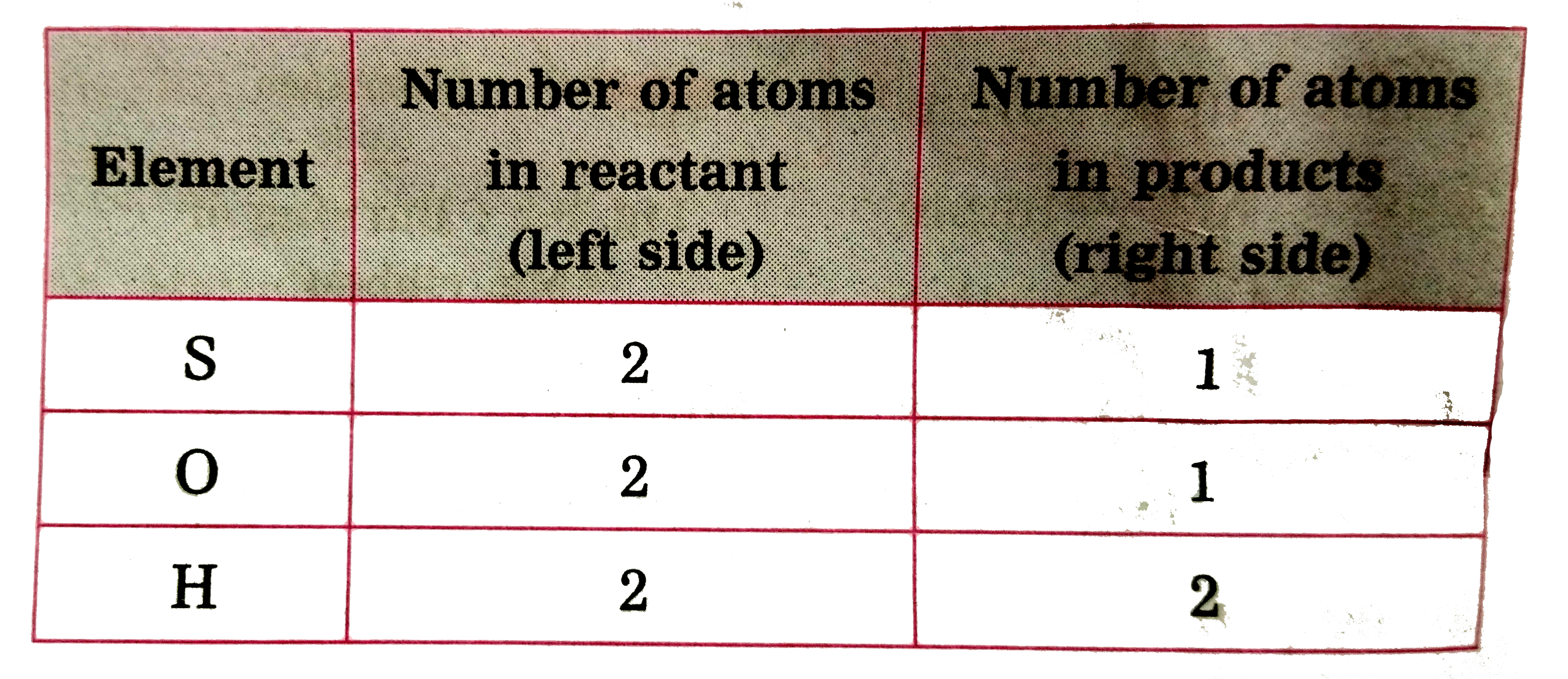
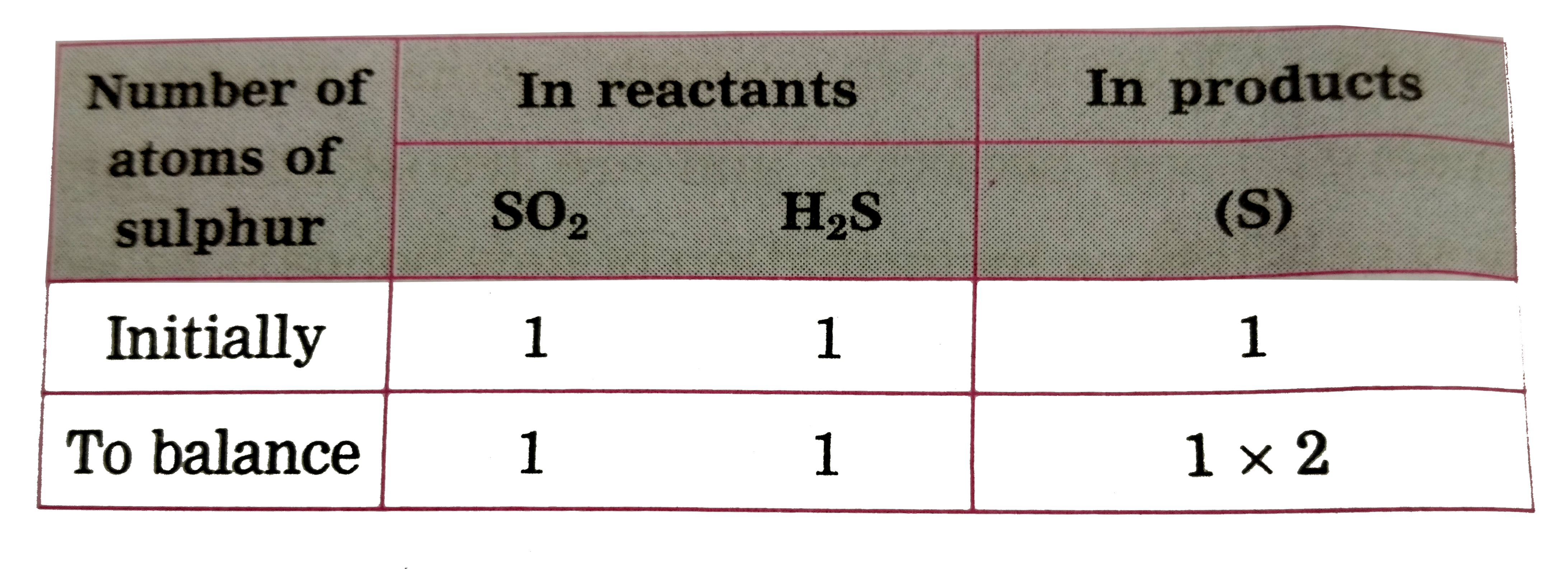
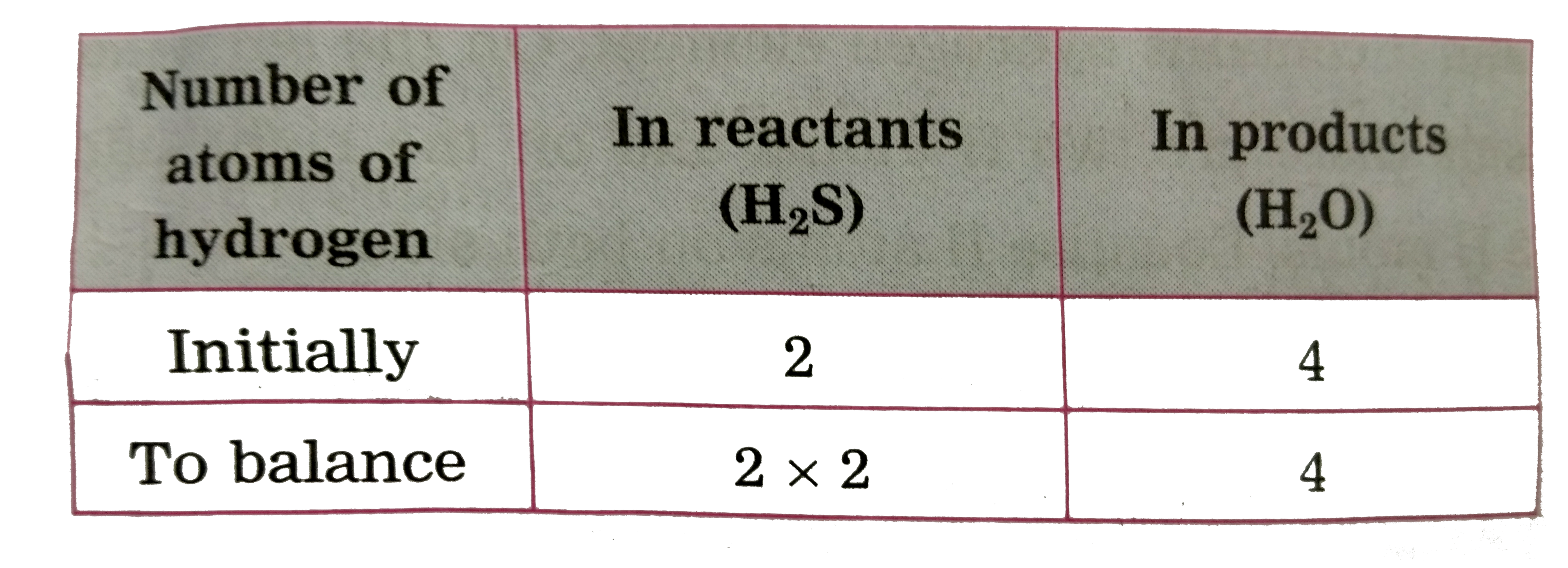
 ltbgt To equalise the number of sulphur atoms, we use 2 as the factor in the product i .e `H_(2)O` , now the unbalanced equation becomes
ltbgt To equalise the number of sulphur atoms, we use 2 as the factor in the product i .e `H_(2)O` , now the unbalanced equation becomes