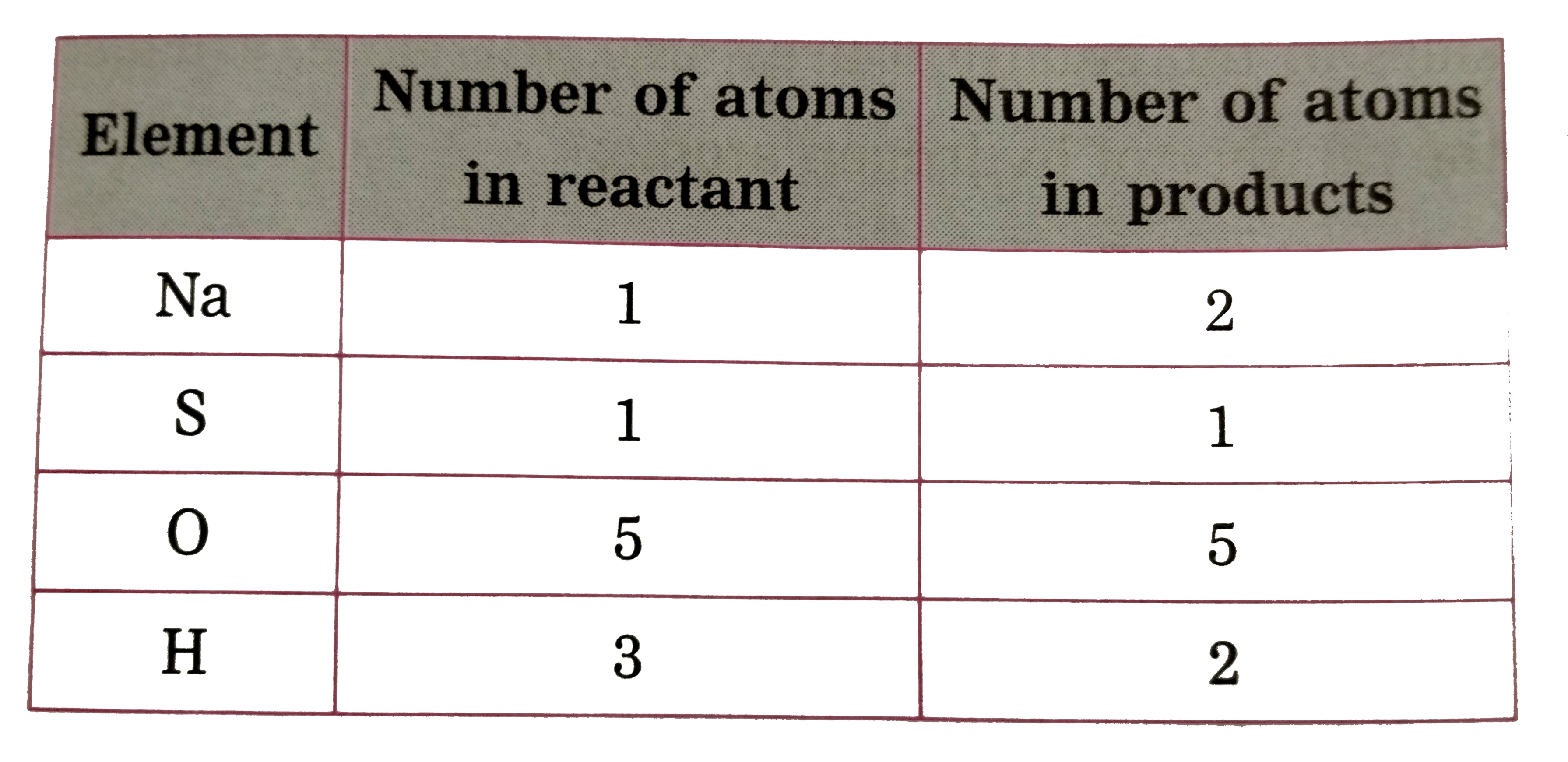
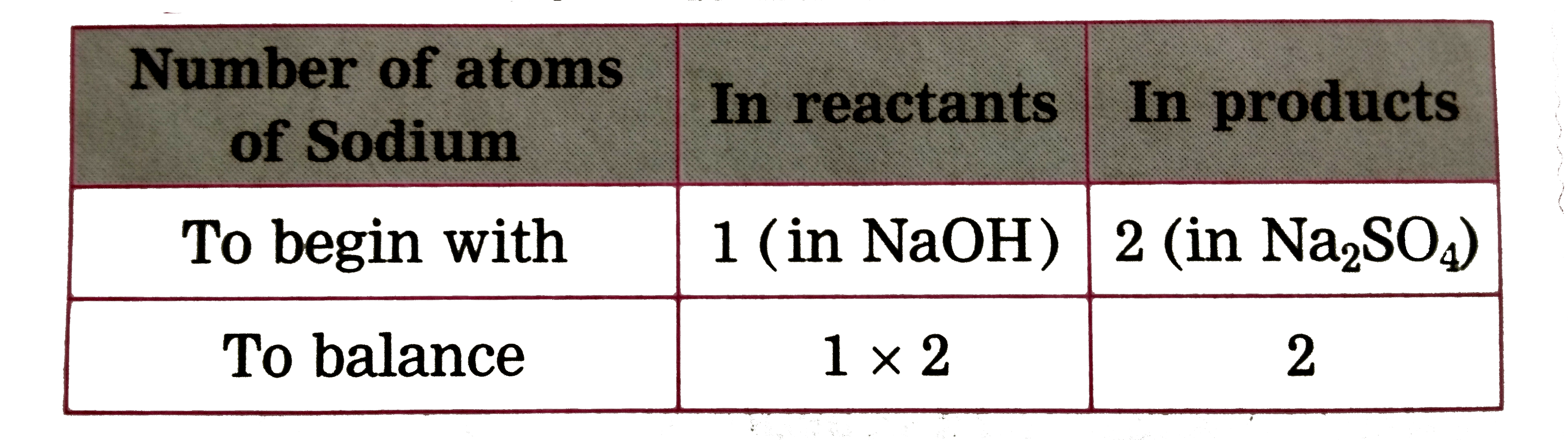Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Write the balanced equations for the following reactions : * (iv) H(2...
Text Solution
|
- Complete the following chemical reaction equations : (i)P (4(s)) +Na...
Text Solution
|
- Predict the outcome of the following reactions and write balanced equa...
Text Solution
|
- The reaction NaOH((s)) + H(2)O((I)) to NaOH((aq)) is a/an ………… reactio...
Text Solution
|
- (iii) H(2)SO(4(aq)) + NaOH((aq)) to Na(2)SO(4(aq)) + H(2)O((I))
Text Solution
|
- The reaction CuSO(4((aq)))+Zn(s)rarrZnSO(4((aq)))+Cu(s) is a ………….. re...
Text Solution
|
- CuSO(4((aq)))+Zn((s))rarrZnSO(4((aq)))+Cu((s)) is example of decomposi...
Text Solution
|
- Write the balanced equations for the following reactions : * (i) H(2)S...
Text Solution
|
- Write the balanced equations for the following reactions : * (ii) SO(2...
Text Solution
|