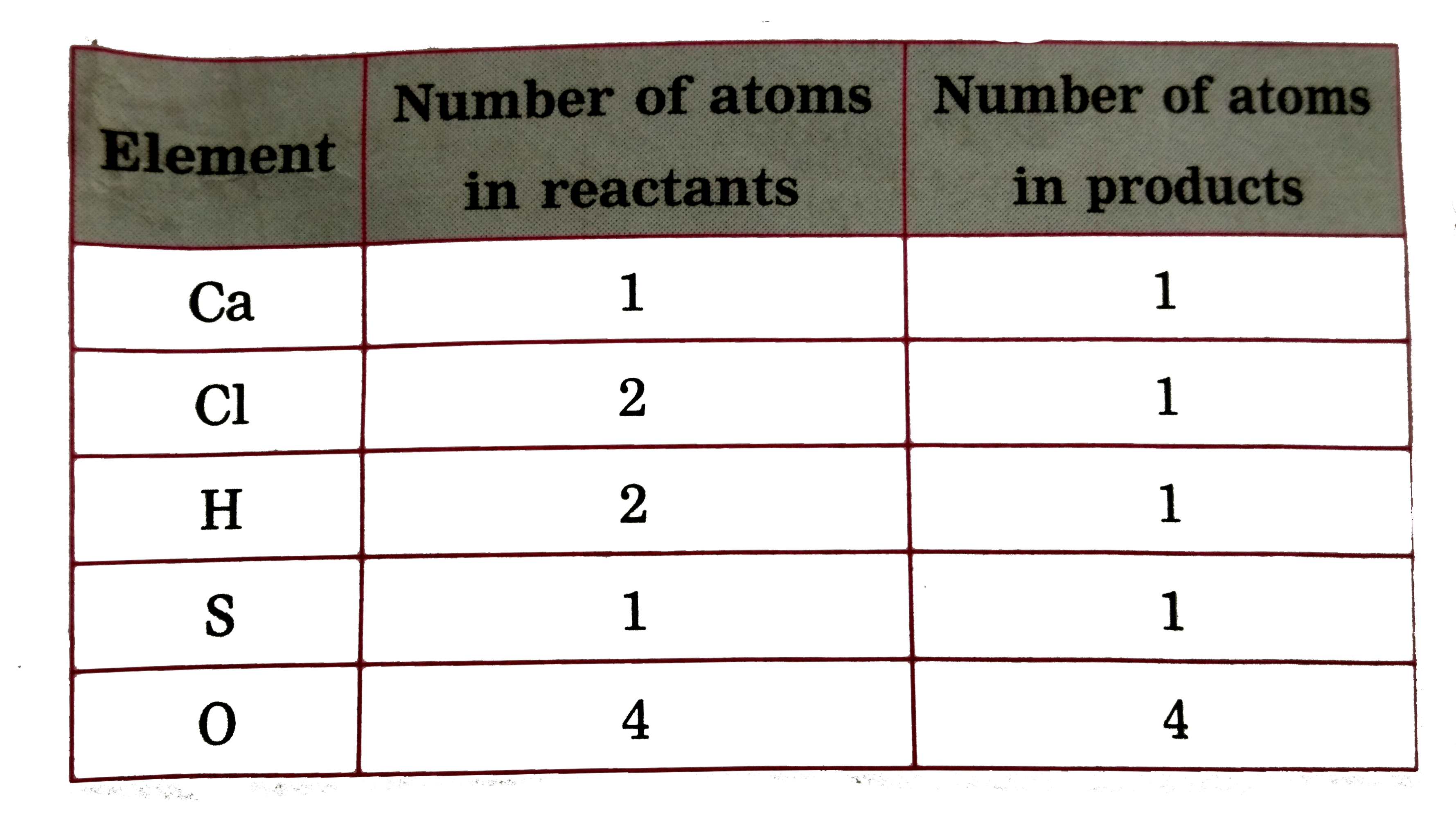
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Write the balanced equations for the following reactions : (vi) Cal...
Text Solution
|
- Write down a balanced chemical equation for the following reaction. ...
Text Solution
|
- Write the balanced equations for the following reactions : (vi) Calciu...
Text Solution
|
- Balance the equation stepwise. Calcium chloride + Sulphuric acid to ca...
Text Solution
|
- Write down a balanced chemical equation for the following reaction: Ca...
Text Solution
|
- Write down a balanced chemical equation for the following reaction: Ca...
Text Solution
|
- अभिक्रिया की सन्तुलित रासायनिक समीकरण लिखिए- कैल्सियम कार्बोनेट + हा...
Text Solution
|
- निम्नलिखित रासायनिक अभिक्रियाओं के लिए सन्तुलित समीकरण लिखिए- बेरियम...
Text Solution
|
- निम्न अभिक्रियाओं के लिए संतुलित रासायनिक समीकरण लिखिए - (अ) कैल्सिय...
Text Solution
|