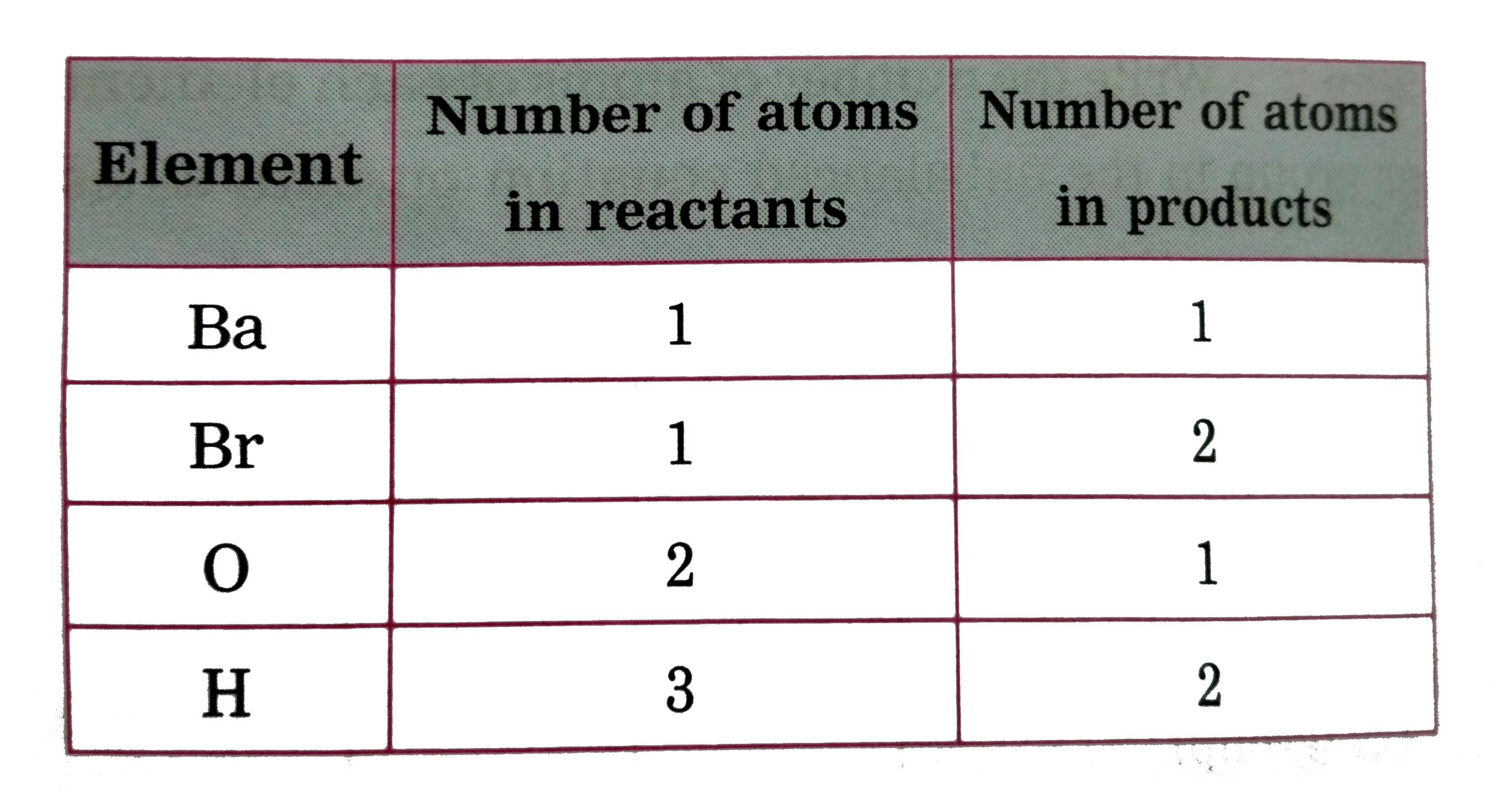
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Write the balanced equations for the following reactions : (vii) Ba...
Text Solution
|
- The following reaction is a/an Ba(OH)(2)+H(2)SO(4) to BaSO(4)+2H(2)O
Text Solution
|
- निम्न क्षारकों को प्रबलता के घटते कर्म में लिखिए। NH(4)OH, NaOH,H(2)...
Text Solution
|
- Write the balanced equations for the following reactions : CH(4)+O(2...
Text Solution
|
- क्या निम्नलिखित अभिक्रिया संतुलित है? C(2)H(5)OH+2O(2)to 2CO(2)+3H(2...
Text Solution
|
- Write the balanced equations for the following reactions : (vii) Ba(OH...
Text Solution
|
- Write the balanced equations for the following reactions : CH(4)+O(2...
Text Solution
|
- Balance the following equations. 3) Mg(OH)(2)+HCl rarr MgCl(2)+H(2)O
Text Solution
|
- निम्नलिखित रेडॉक्स अभिक्रिया को सन्तुलित कीजिए - Cl(2) + OH^(-)toCl^...
Text Solution
|