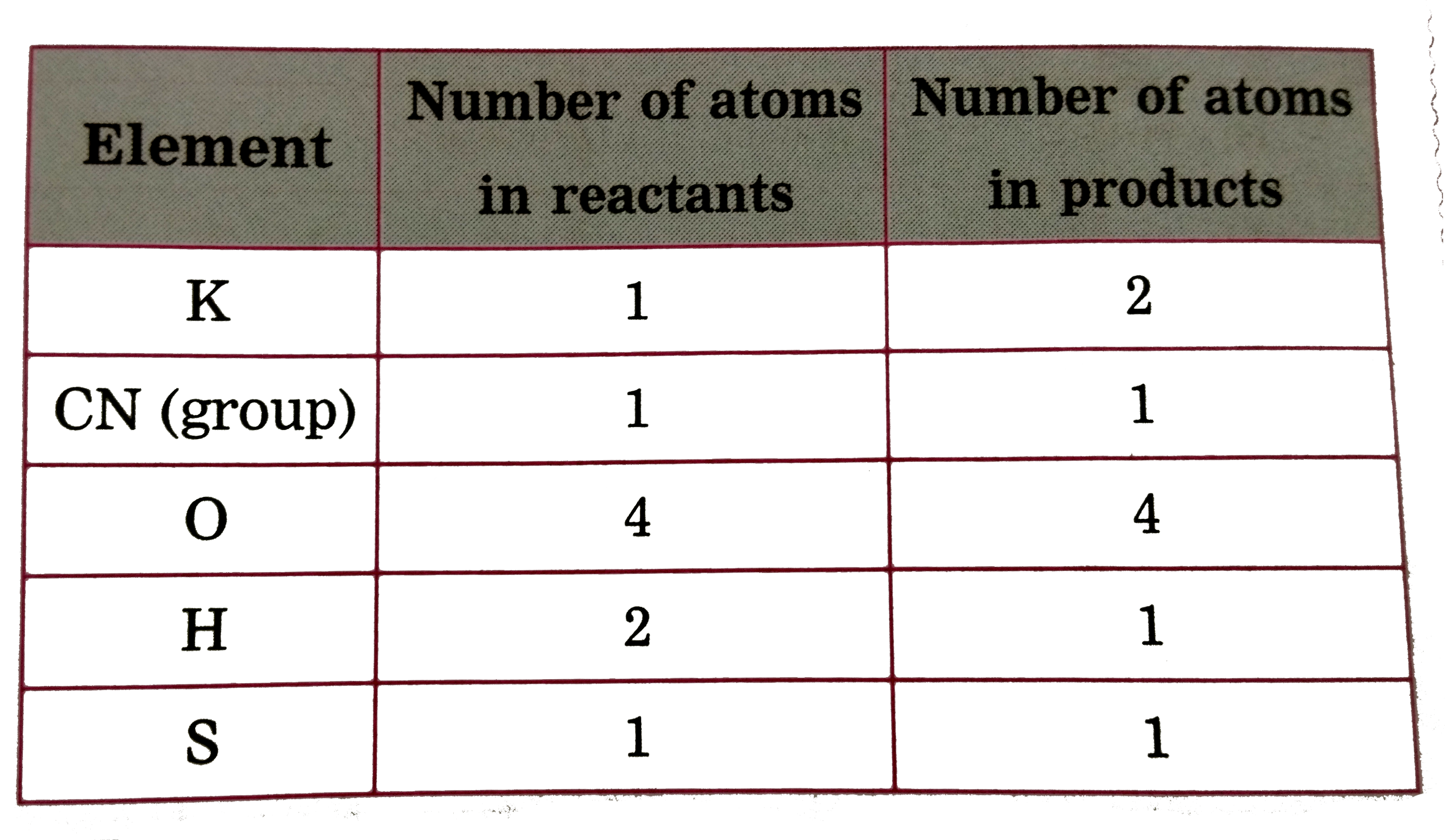
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Write the balanced equations for the following reactions : (viii) KCN+...
Text Solution
|
- Balance the following equation by oxidation number method Al+KMnO(4...
Text Solution
|
- समीकरण Al + H(2)SO(4) rarr Al(2)(SO(4))(3) + H(2) को संतुलित करना ।
Text Solution
|
- Write the balanced equations for the following reactions : (viii) KCN+...
Text Solution
|
- Balance the following equations by oxidation number method K(2)Cr(2)...
Text Solution
|
- Balance the following equations by oxidation number method KMnO(4)+H...
Text Solution
|
- Write balanced equations for the following reactions: Carbon reacts wi...
Text Solution
|
- Balance the following equation by oxidation number method. KMnO(4)+FeS...
Text Solution
|
- Balanced the following chemical equations : NaOH+H(2)SO(4)toNa(2)SO(...
Text Solution
|