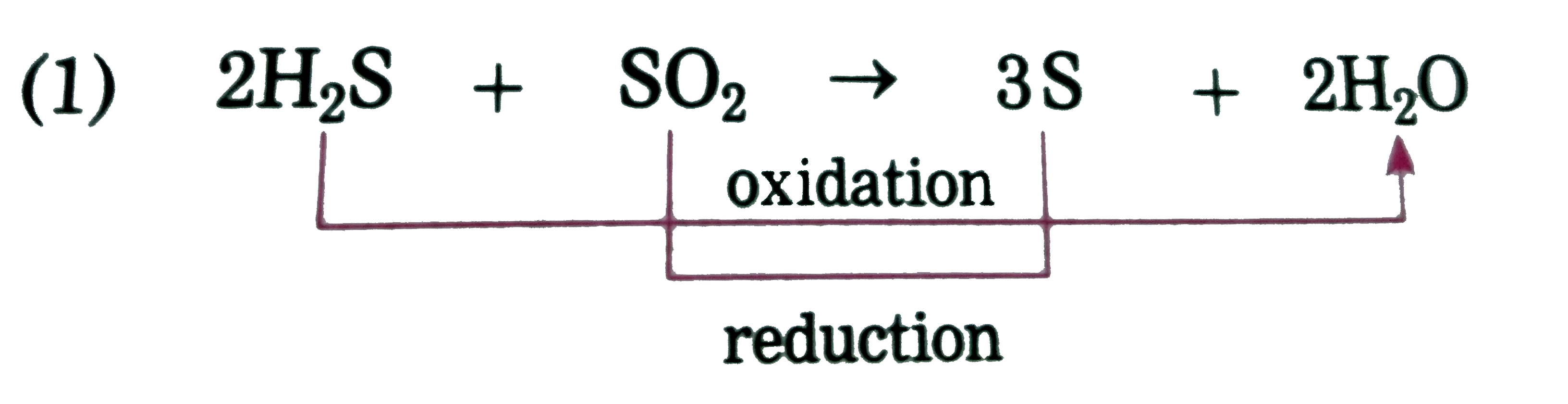
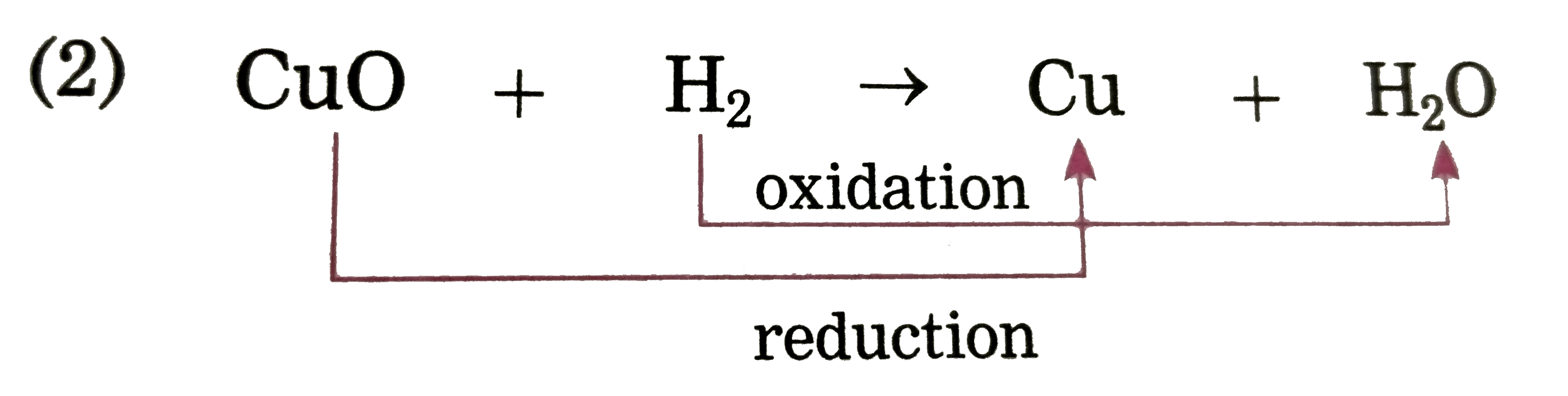
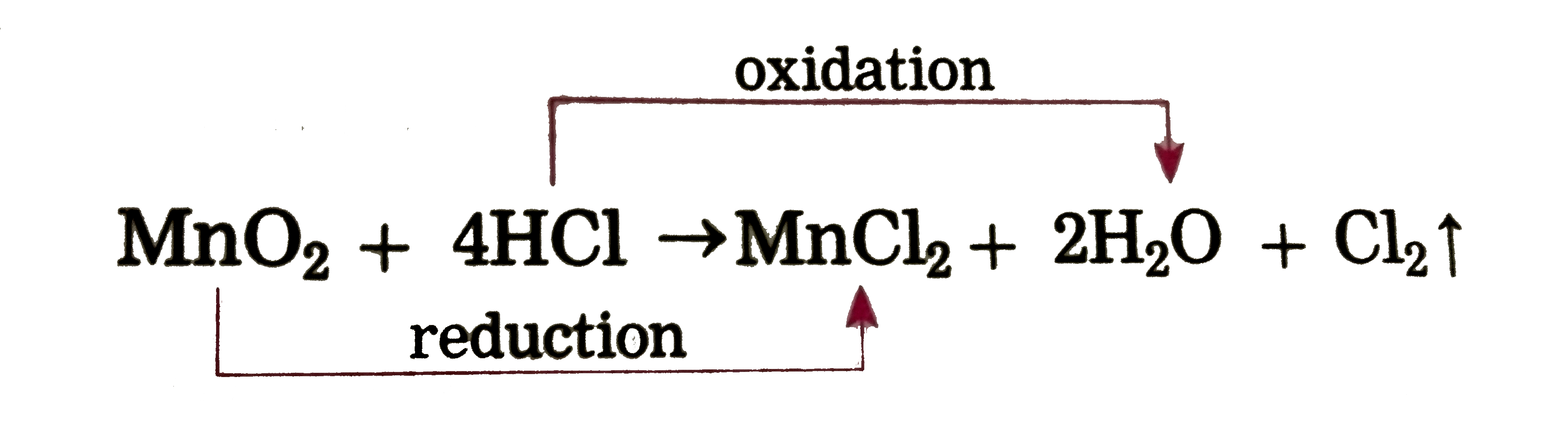Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- What are redox reactions ? Identify the substances that are oxidised a...
Text Solution
|
- In the following reaction, 2H(2)S(g)+SO(2)(g) rarr 3S(s)+2H(2)O(l) One...
Text Solution
|
- Given chemical equations for these reactions S(s)+O(2)(g)toSO(2)(g)D...
Text Solution
|
- Identify the substances that are oxidised and the substances that are ...
Text Solution
|
- Identify the substances that are oxidised and the substances that are ...
Text Solution
|
- Identify the substances that are oxidised and the substances that are ...
Text Solution
|
- Identify the substances that are oxidised and the substances that are ...
Text Solution
|
- निम्नलिखित अभिक्रिया में H(2)S तथा SO(2) में S की ऑक्सीकरण संख्या में ...
Text Solution
|
- What are redox reactions ? Identify the substances that are oxidised a...
Text Solution
|