Text Solution
Verified by Experts
Recommended Questions
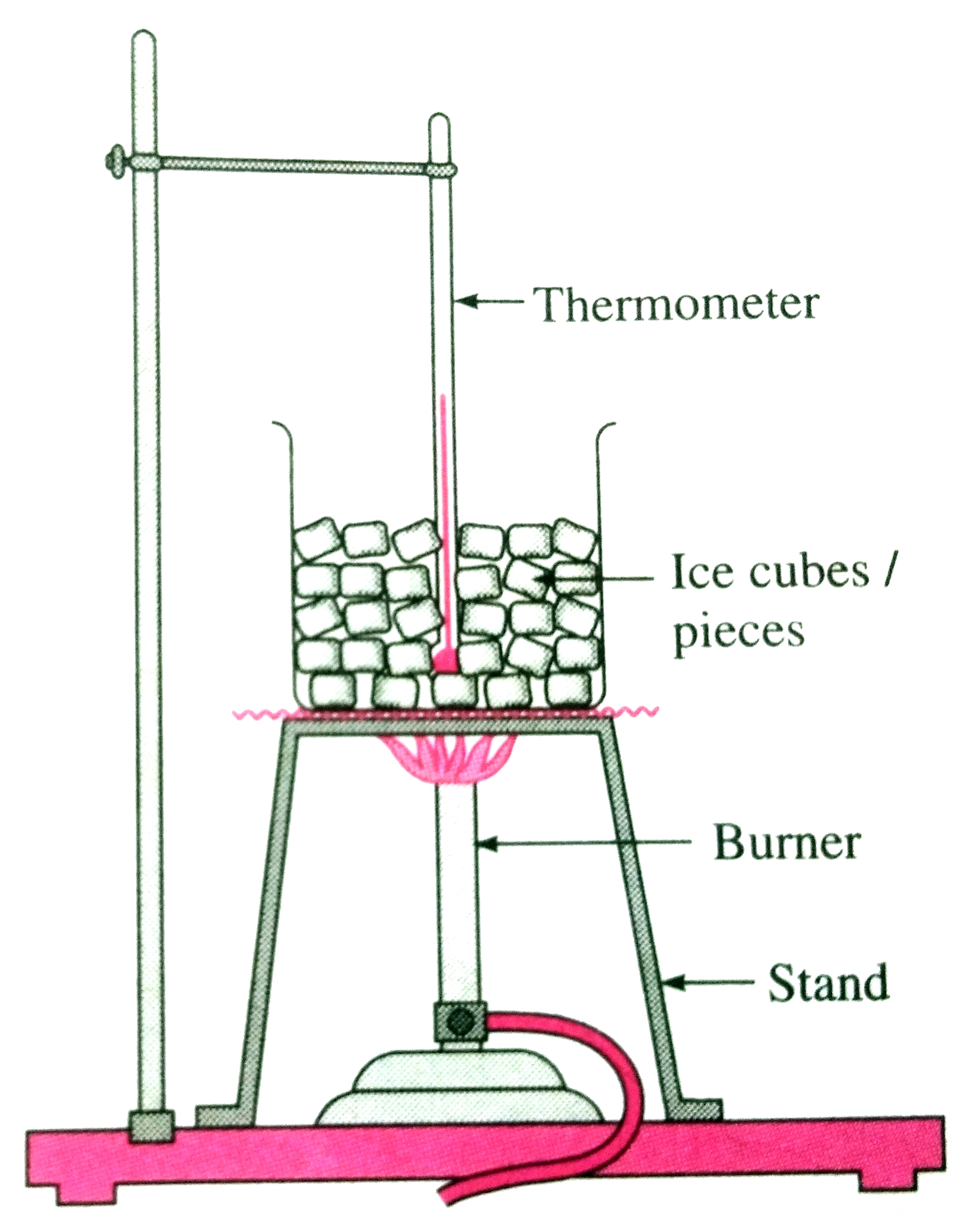
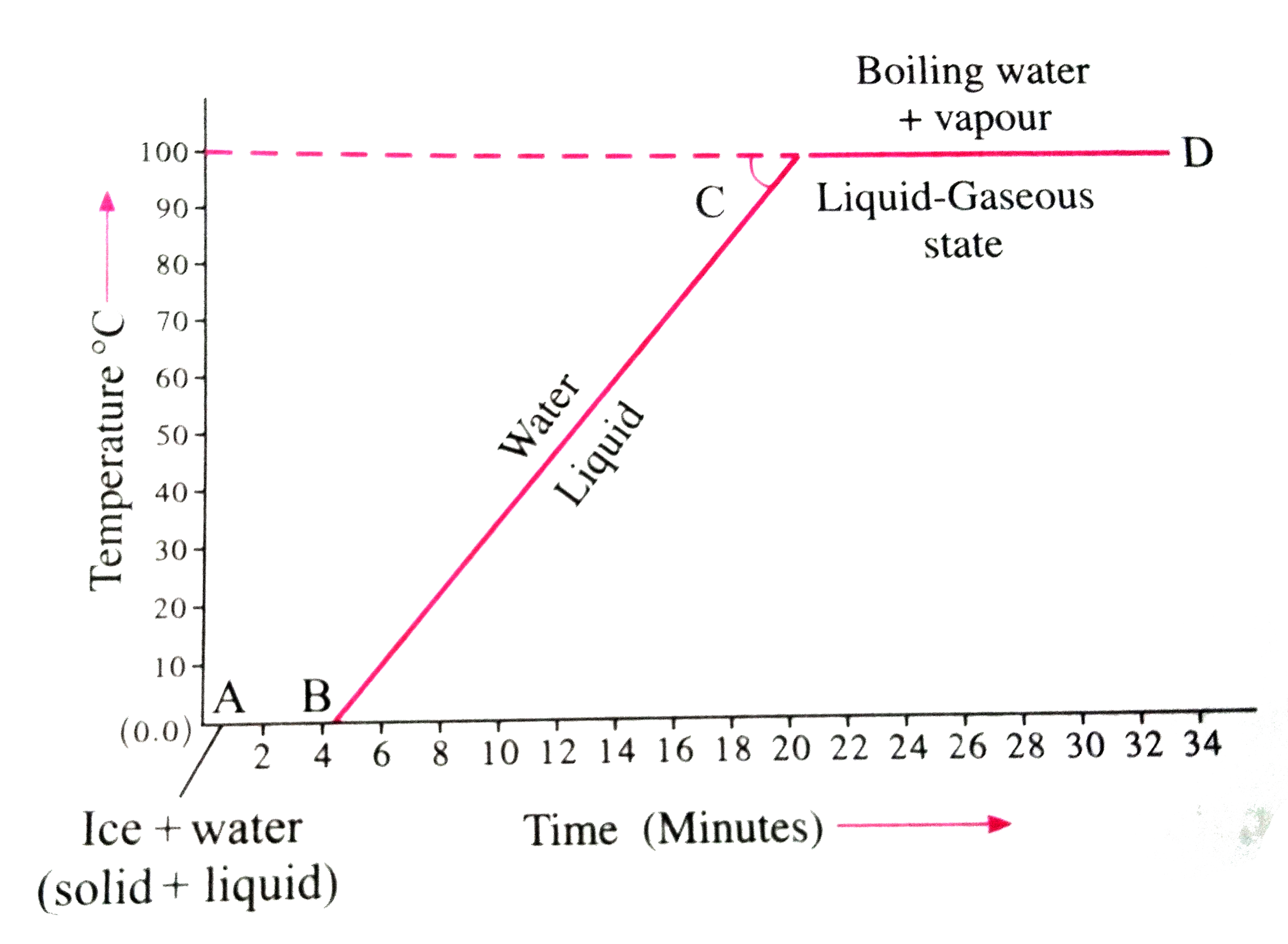
- (1) Take a few pieces of ice in a glass beaker as shown in figure. ...
Text Solution
|
- (1) Take a few pieces of ice in a glass beaker as shown in figure. (2)...
Text Solution
|
- (1) Take a few pieces of ice in a glass beaker as shown in figure. ...
Text Solution
|
- (1) Take a few pieces of ice in a glass beaker as shown in figure. ...
Text Solution
|
- (1) Take a few pieces of ice in a glass beaker as shown in figure. ...
Text Solution
|
- (1) Take a few pieces of ice in a glass beaker as shown in figure. ...
Text Solution
|
- (1) Take a few pieces of ice in a glass beaker as shown in figure. ...
Text Solution
|
- (1) Take a few pieces of ice in a glass beaker as shown in figure. (2)...
Text Solution
|
- (1) Take a few pieces of ice in a glass beaker as shown in figure. (2)...
Text Solution
|