Text Solution
Verified by Experts
Topper's Solved these Questions
HEAT
NAVNEET PUBLICATION - MAHARASHTRA BOARD|Exercise NUMERICAL PROBLEMS FOR PRACTICE|12 VideosHEAT
NAVNEET PUBLICATION - MAHARASHTRA BOARD|Exercise Textbook page 65|6 VideosGRAVITATION
NAVNEET PUBLICATION - MAHARASHTRA BOARD|Exercise Solve the following examples/numerical problems :|37 VideosLAWS, RULES, UNITS AND DEFINITIONS
NAVNEET PUBLICATION - MAHARASHTRA BOARD|Exercise Carbon Compounds|18 Videos
Similar Questions
Explore conceptually related problems
NAVNEET PUBLICATION - MAHARASHTRA BOARD-HEAT-Textbook page 66
- Define relative humidity . OR What is relative humidity ? Wri...
Text Solution
|
- On what basis and how will you determne whether air is saturated with ...
Text Solution
|
- What is the value of relative humidity at the dew point temperature ?
Text Solution
|
- The mass of water vapour in air enclosed in a certain space 60g and th...
Text Solution
|
- During winter, sometimes we see a white trail at the back of a flying ...
Text Solution
|
- State two effects of humidity present in atmosphere.
Text Solution
|
- Explain how dew and fog are formed. OR Write a short note on format...
Text Solution
|
- How can you relate the formation of water droplets on the outer surfac...
Text Solution
|
- State the units of heat .
Text Solution
|
- Define the kilocalorie.
Text Solution
|
- Define the calorie.
Text Solution
|
- State the relation between the kilocalorie and the calorie.
Text Solution
|
- While deciding the unit for heat, which temperature interval is chose...
Text Solution
|
- What is mean by specific heat capacity ? [OR Define specific heat cap...
Text Solution
|
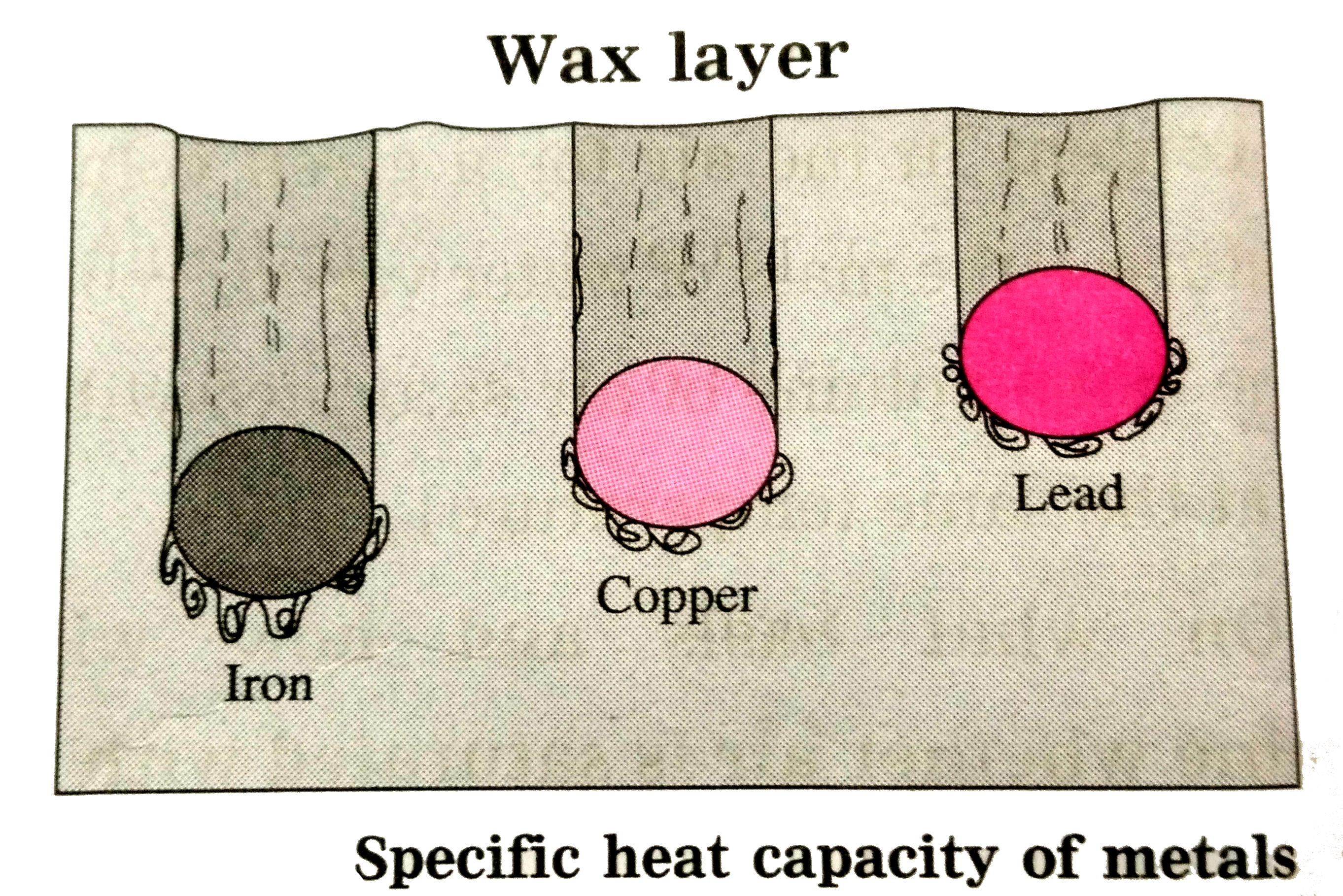
- Study the following procedure and answer the question below : 1. Ta...
Text Solution
|
- Write the symbol for specific heat capacity . State the units of spec...
Text Solution
|
- Which principle is used to measure the specific heat capacity of a sub...
Text Solution
|
- Principle of heat exchange:
Text Solution
|
- The specific heat capacity of silver is 0.056kcal //kg.^(@)C . Expla...
Text Solution
|
- Explain how the specific heat capacity of a solid can be determined ( ...
Text Solution
|