Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
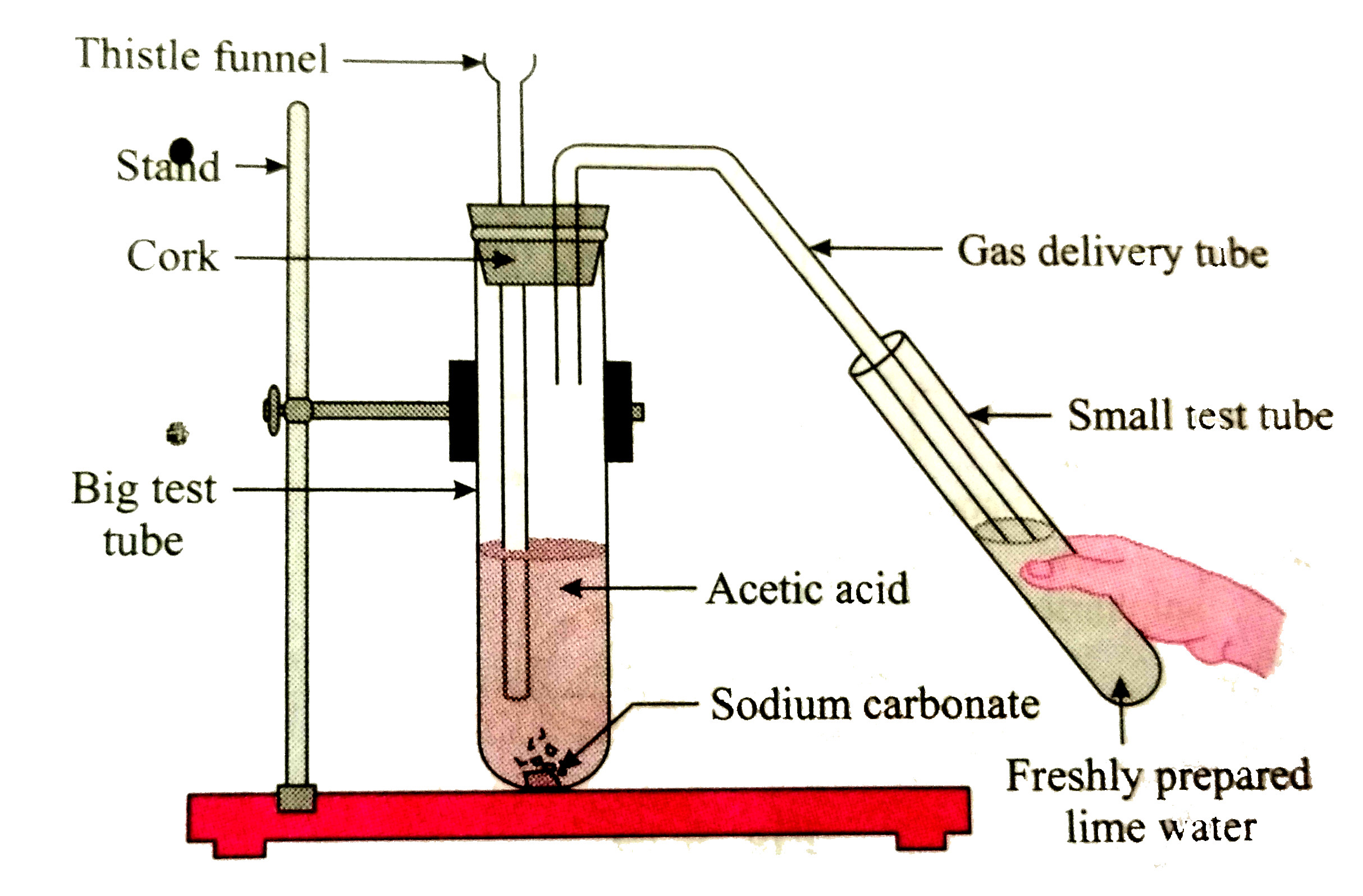
- Apparatus: Big test tube, small test tube, bent gas delivery tube, rub...
Text Solution
|
- Apparatus: Big test tube, small test tube, bent gas delivery tube, rub...
Text Solution
|
- Apparatus: Big test tube, small test tube, bent gas delivery tube, rub...
Text Solution
|
- Apparatus: Big test tube, small test tube, bent gas delivery tube, rub...
Text Solution
|
- Apparatus: Two test tubes, bent tube, rubber cork, burner, etc. Chemic...
Text Solution
|
- Apparatus: Two test tubes, bent tube, rubber cork, burner, etc. Chemic...
Text Solution
|
- Apparatus: Two test tubes, bent tube, rubber cork, burner, etc. Chemic...
Text Solution
|
- Apparatus: Two test tubes, bent tube, rubber cork, burner, etc. Chemic...
Text Solution
|
- Apparatus: Big test tube, delivery tube fitted in a rubber cork, knife...
Text Solution
|