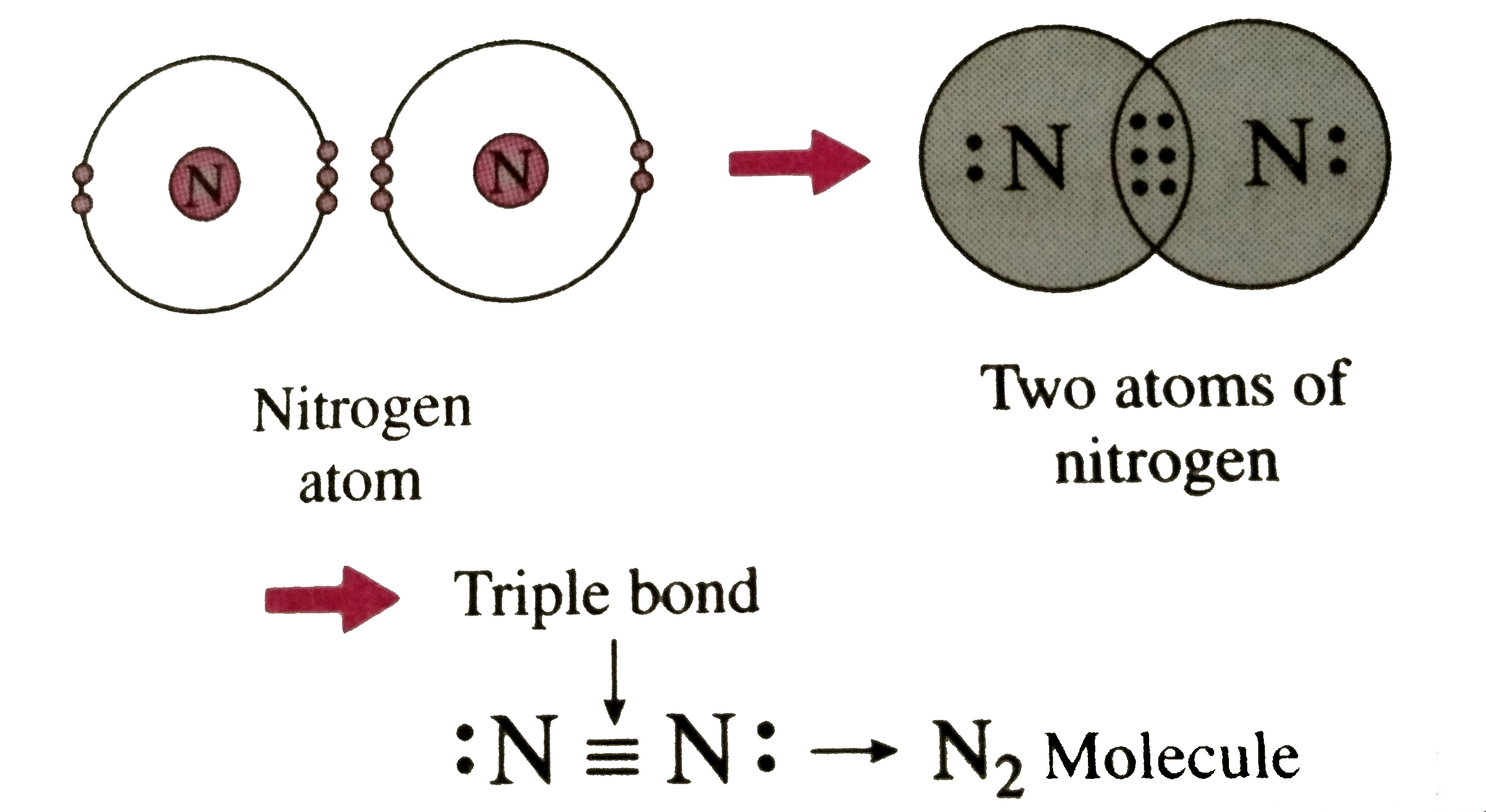Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
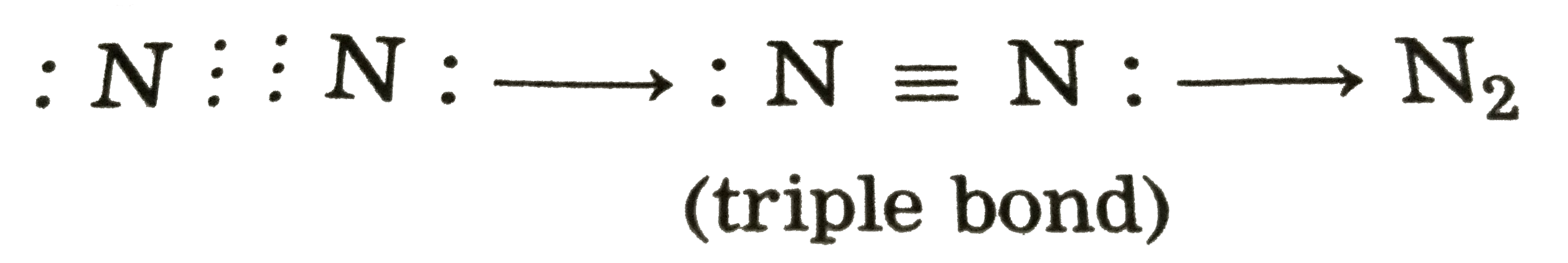
- Describe the formation of nitrogen molecule.
Text Solution
|
- Explain formation of covalent bond by the electron structure of hydrog...
Text Solution
|
- Explain the formation of hydrogen, oxygen and nitrogen molecules.
Text Solution
|
- Number of electrons shared in the formation of nitrogen molecules i...
Text Solution
|
- SO(3) अणु के निर्माण तथा संरचना का वर्णन कीजिए ।
Text Solution
|
- Describe the formation of oxygen molecule (O(2)).
Text Solution
|
- Describe the formation of nitrogen molecule.
Text Solution
|
- How many pairs of electrons are involved in bond formation in case of ...
Text Solution
|
- In the formation of nitrogen molecule.
Text Solution
|