A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
P BAHADUR-ATOMIC STRUCTURE-Exercise 3A
- What is the difference in the angular momentum associated with the e...
Text Solution
|
- Ionisation potential fo hydrogen atomn is 13 . 6 eV. Hydrogen atom in...
Text Solution
|
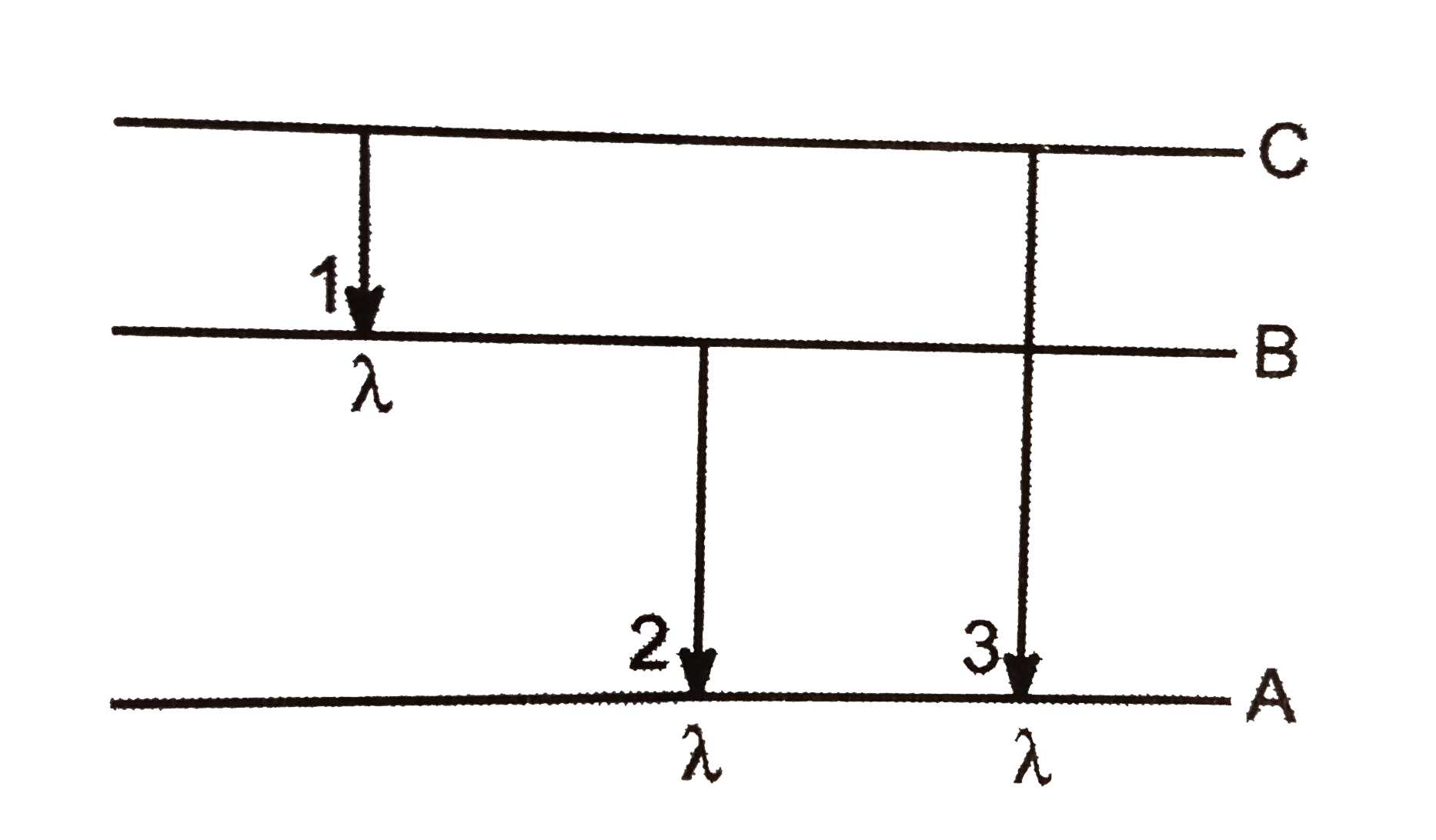
- Energy levels A. B, C. of a certain atom correspoinds to increasing v...
Text Solution
|
- One requiored enrgy R(n) to remove mucleon and an energy Ee to remov...
Text Solution
|
- Atomic radius is of the order of 10^(-8) cm and nuclear radius is o...
Text Solution
|
- Suppose 10^(-17) J of light enrgy is needed by the interor of human e...
Text Solution
|
- A photon of 300 nm ios absorbed by a gas and then re-emits two photon...
Text Solution
|
- The shortest wavelength in H sopecitrum of lyman series when R(H) = 1...
Text Solution
|
- The longest lambda for the Lyamn series is …………… (Given RH = 109678 ...
Text Solution
|
- Number of elrctrons in 1. 8 mL of H2 O are :
Text Solution
|
- The total number of electrons presnt in 1 mL Mg Given density of ...
Text Solution
|
- What transition in He^(o+) ion shall have the same wave number as ...
Text Solution
|
- Three isotopes fo an elecment have mass numbers (m, (m + 1) and (M +...
Text Solution
|
- The radii of two of the first four Bohre's orbits of the hydrogen atom...
Text Solution
|
- When photon of energy 25eV strike the surface of a metal A, the ej...
Text Solution
|
- If the total enrgy fo an electron in a hydrogen linke atom in an ecite...
Text Solution
|
- The highest excited state that unexcited hydrogen atom can ereach when...
Text Solution
|
- The approximate quantum number fo a circular orbit of diamere , 20, n...
Text Solution
|
- A boll of mass 200 g is moving with a velocity of 10 m sec^(-1) . If ...
Text Solution
|
- p-orbitals fo an atom in presence fo magnetic field are :
Text Solution
|
 .
.