A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
P BAHADUR-ATOMIC STRUCTURE-Exercise 8
- Statement : The 3p-orbital has higher energy level than 3s in He^(+) i...
Text Solution
|
- Statement : Specific charge of alpha-particles is twice to that of pr...
Text Solution
|
- Statement : d-orbital are five fold non-degenerate in presence of magn...
Text Solution
|
- Statement : electromangetic radiations will be emitted for the transti...
Text Solution
|
- Statement : The psi(640) represents an orbital . Explanation : The o...
Text Solution
|
- Statement : Monochromatic X-rays fall on lighter elements such as carb...
Text Solution
|
- Statement : .(24)Cr has more paramangetic nature than .(25)Mn. Expl...
Text Solution
|
- Statement : The possible number of electrons in a subshell is (4l + 2)...
Text Solution
|
- Statement : Aufbau rule is violated in writing electronic configuratio...
Text Solution
|
- Humphry series discovered in H-"atomic" spectra has lowest energy rad...
Text Solution
|
- Assertion (A) : Hydrogen has only one electron in its 1s orbital but i...
Text Solution
|
- Statement : Wave number of a spectral line for an electronic transitio...
Text Solution
|
- Statement : The tendency of a atom to reach a stable electronic arrang...
Text Solution
|
- Statement : For n=3,l may be 0,1 and 2, and (m) may be 0 , 0, +- ...
Text Solution
|
- Statement : Number of waves in an orbit of atom is equal to number of ...
Text Solution
|
- Statement : wavelength of (I) line of Humphry series is more than (I) ...
Text Solution
|
- Statement : All s-orbitla in H-atom corresponds to a non-zero probabil...
Text Solution
|
- Statement : The energy radiated per unit volume , i.e., energy density...
Text Solution
|
- Statement : The plot of atomic number ( y -axis ) versus number of neu...
Text Solution
|
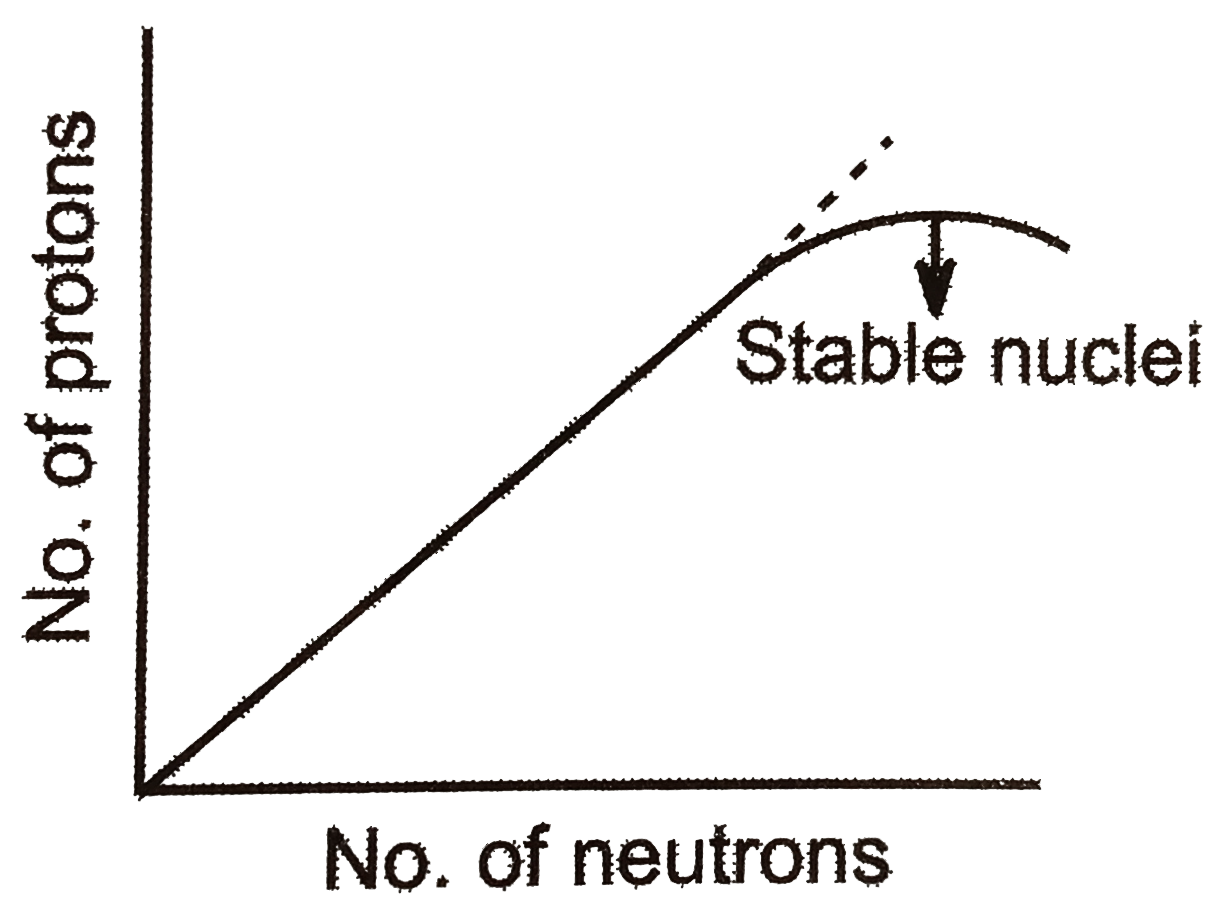 ltbr. If the curve does not bend down towards the x-axis then the proton -proton repulison would overcome the attractive forces of proton and neutron. Therefore, curve bends down to achieve stability of the between proton and neutron overcome proton -proton electrostatic repulsion.
ltbr. If the curve does not bend down towards the x-axis then the proton -proton repulison would overcome the attractive forces of proton and neutron. Therefore, curve bends down to achieve stability of the between proton and neutron overcome proton -proton electrostatic repulsion.