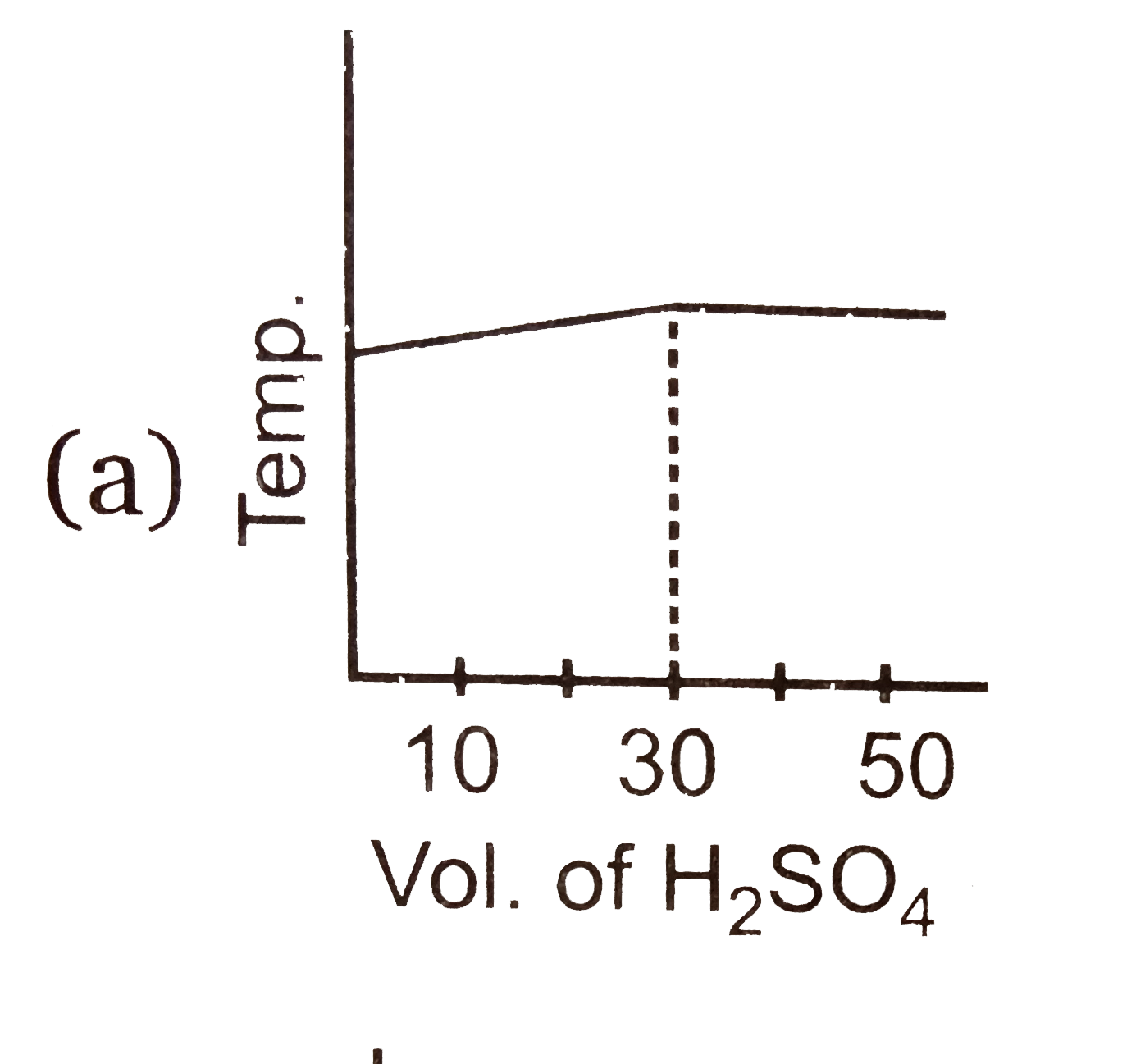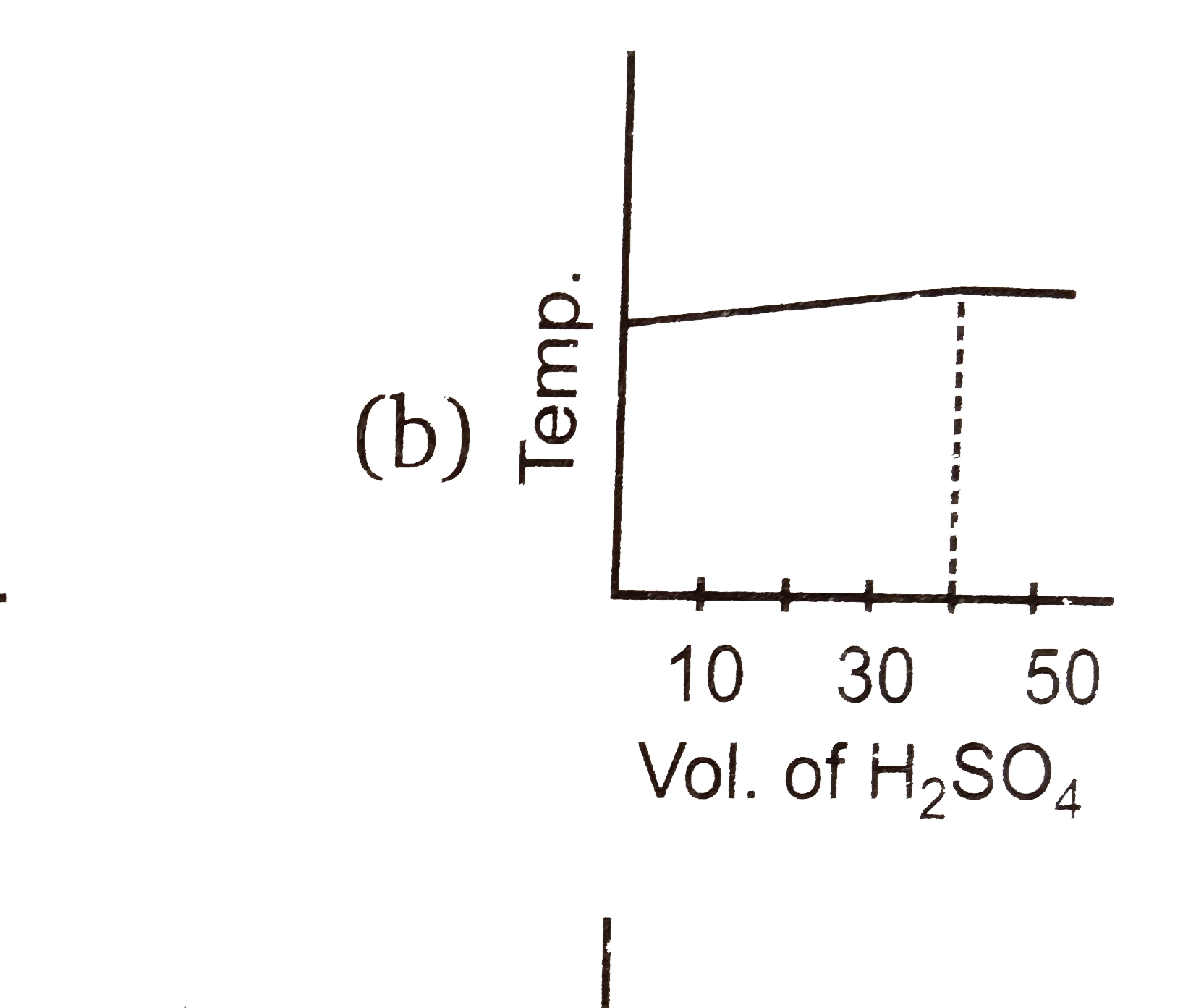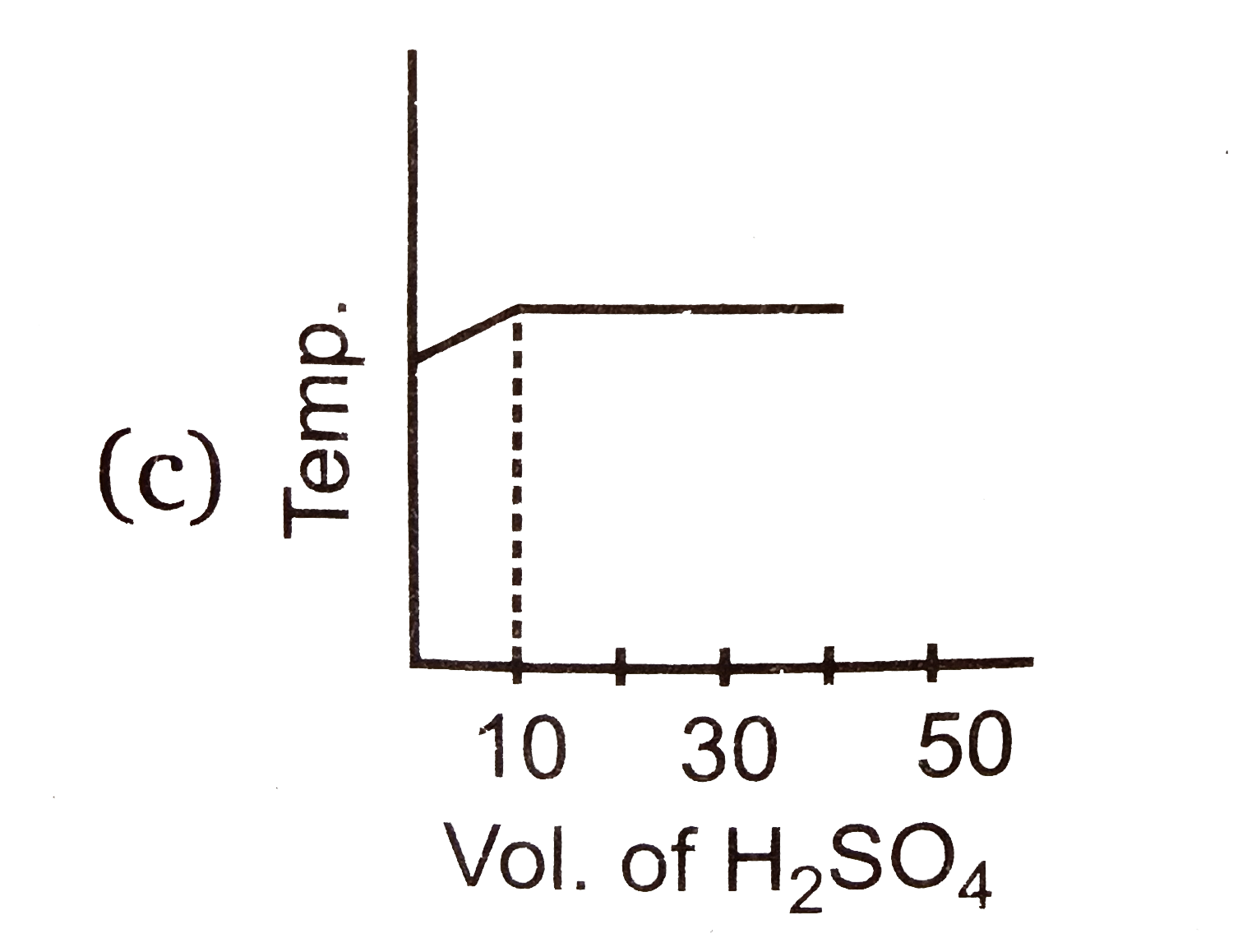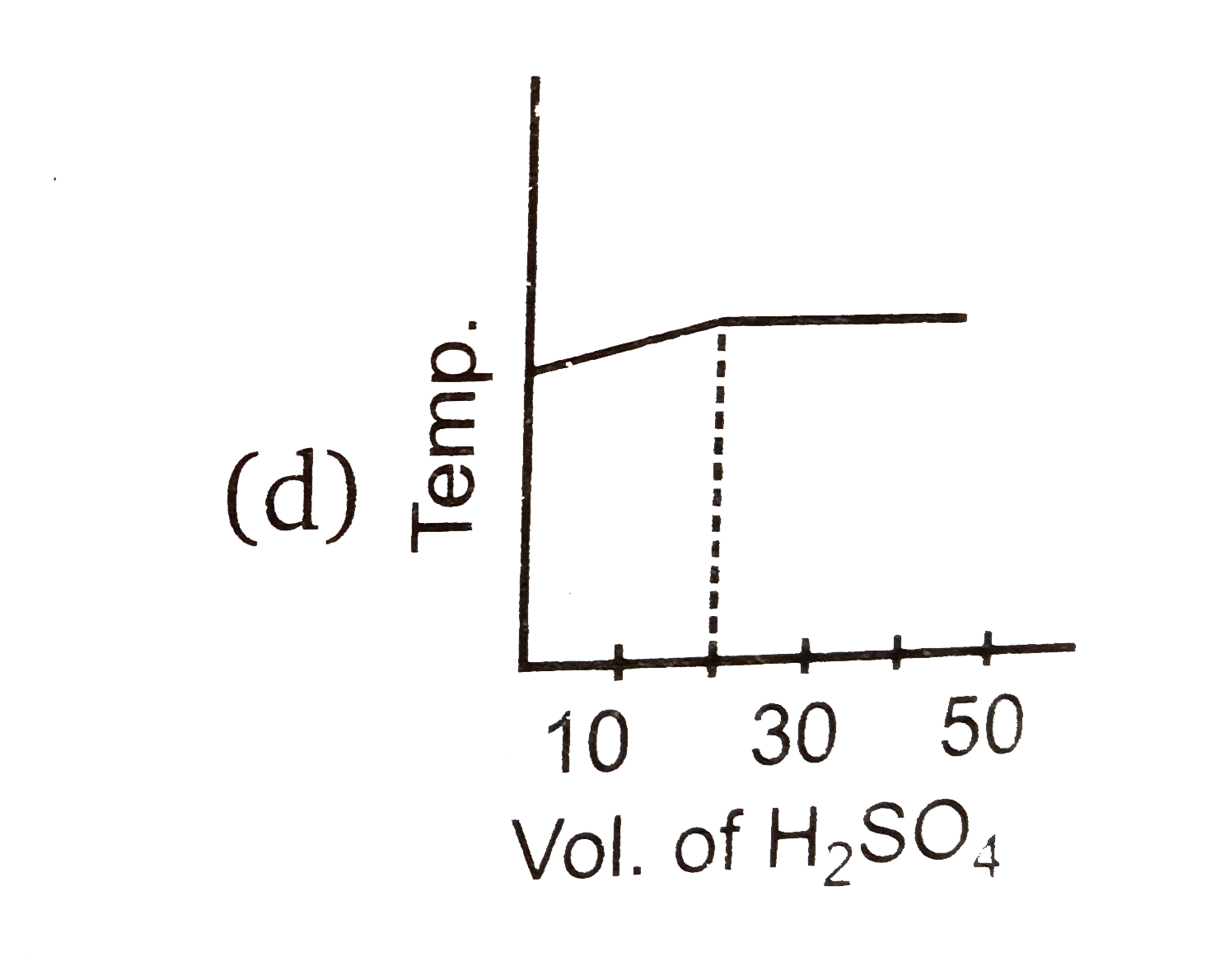A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
IONIC EQUILIBRIUM
P BAHADUR|Exercise Exercise3B|30 VideosIONIC EQUILIBRIUM
P BAHADUR|Exercise Exercise4|32 VideosIONIC EQUILIBRIUM
P BAHADUR|Exercise Exercise2|52 VideosGASEOUS STATE
P BAHADUR|Exercise Exercise -9|1 VideosMOLE AND EQUIVALENT CONCEPT
P BAHADUR|Exercise Exercise 9 Advanced numerical problems|61 Videos
Similar Questions
Explore conceptually related problems
P BAHADUR-IONIC EQUILIBRIUM-Exercise3A
- The solubility products of AI(OH)(3) and Zn(OH)(2) are 8.5xx10^(-23) a...
Text Solution
|
- The solubilituy of PbCI(2) in water is 0.01M 25^(@)C. Its maximum conc...
Text Solution
|
- In an experiment to determine the enthalpy of neutralisation of sodium...
Text Solution
|
- Calculate K for the reaction, A^(-)+H(3)^(+)O hArr HA+H(2)O if K(a) ...
Text Solution
|
- The degree of hydrrolysis of a salt of weak acid and weak base in its ...
Text Solution
|
- The volume of the water needed to dissolve 1 g of BaSO(4) (K(SP)=1.1xx...
Text Solution
|
- Let the solubilities of AgCI in H(2)O, and in 0.01M CaCI(2), 0.01M NaC...
Text Solution
|
- From separate solutions of sodium salts, NaW, NaX, NaY and NaZ have pH...
Text Solution
|
- A cetrain ion B^(-) has an Arrhenius constant for basic character (eq....
Text Solution
|
- Acetic acid and propionic acid have K(a) values 1.75xx10^(-5) and 1.3x...
Text Solution
|
- The ionization constant of overset(o+)(NH(4)) ion in water is 5.6 xx 1...
Text Solution
|
- A solution of Na(2)CO(3) is added drop by drop to litre of a solution ...
Text Solution
|
- To separate and identify the ionis in a mixutre that may contain Pb^(2...
Text Solution
|
- pH of a mixture containing 0.10 M X^(-) and 0.20 M HX is: [pK(b)(X^(-)...
Text Solution
|
- To prepare a buffer of pH 8.26, amount of (NH(4))(2)SO(4) to be added ...
Text Solution
|
- Percentange ionisation of weak acid can be calculated using the formul...
Text Solution
|
- pH of a mixture of 1 M benzoic acid (pK(a)=4.20) and 1 M C(6)H(5)COONa...
Text Solution
|
- If the equilibrium constant for the reaction of weak acid HA with stro...
Text Solution
|
- For, H(3)PO(4)+H(2)OhArrH(3)O^(+)+H(2)PO(4)^(-), K(a(1)) H(2)PO(4)+H...
Text Solution
|
- pH of 0.01 M HS^(-) will be:
Text Solution
|