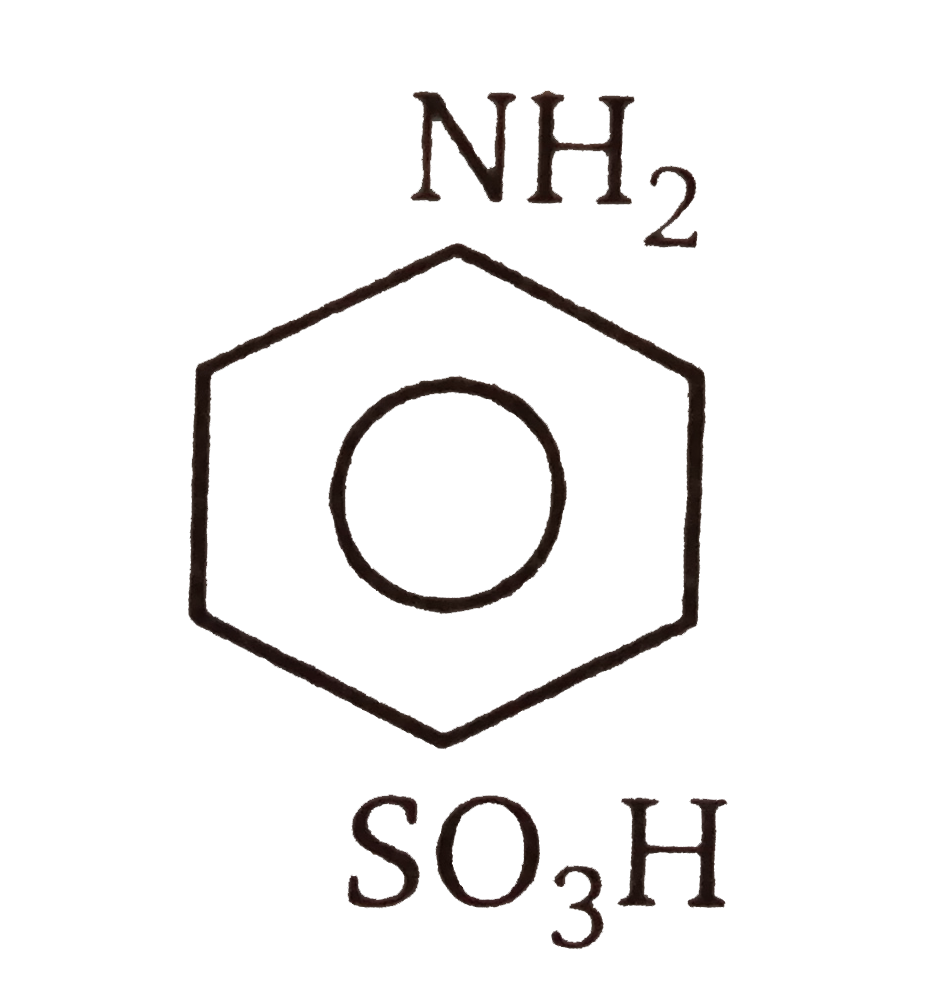
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
IONIC EQUILIBRIUM
P BAHADUR|Exercise Exercise3B|30 VideosIONIC EQUILIBRIUM
P BAHADUR|Exercise Exercise4|32 VideosIONIC EQUILIBRIUM
P BAHADUR|Exercise Exercise2|52 VideosGASEOUS STATE
P BAHADUR|Exercise Exercise -9|1 VideosMOLE AND EQUIVALENT CONCEPT
P BAHADUR|Exercise Exercise 9 Advanced numerical problems|61 Videos
Similar Questions
Explore conceptually related problems
P BAHADUR-IONIC EQUILIBRIUM-Exercise3A
- Which of the following will not produce a precipitate with dilute silv...
Text Solution
|
- 10 mL of a strong acid solution of pH=2.000 are mixed with 990mL of an...
Text Solution
|
- Sulphanilic acid is a/an:
Text Solution
|
- The pH of 10 M HCI solution is:
Text Solution
|
- At infinite dilution, the percentage dissociation of both weak acid an...
Text Solution
|
- Strong acids are generally used as standard solution in acid-base titr...
Text Solution
|
- An acid solution with pH=6at 25^(@)C is diluted by 10^(2) times. The p...
Text Solution
|
- Approximate pH of 0.01M aqueous H(2)S solution, when K(1) and K(2) for...
Text Solution
|
- A student wants to prepared a saturated solution of Ag^(+) ion. He has...
Text Solution
|
- Which of the following species is more soluble in water ?
Text Solution
|
- Number of H^(+) ions present in 10 mL of solution of pH=3 are:
Text Solution
|
- For pure water:
Text Solution
|
- If Delta H("ionisation")^(@) for HCN and CH(3)COOH are 45.2 and 2.1 KJ...
Text Solution
|
- The self ionisation constant for pure HCOOH, K = [HCOO overset(o+)H(2)...
Text Solution
|
- Liquid NH(3) ionises to a slight extent. At -50^(@)C, its ionic produc...
Text Solution
|
- The concentration of fluroacetic acid (K(a)of acid =2.6xx10^(-3)), whi...
Text Solution
|
- The pH of pure water at 25^(@)C and 35^(@)C are 7 and 6, respectively....
Text Solution
|
- The pH of a solution obtained by mixing 10 mL of 0.1MHCI and 40mL of 0...
Text Solution
|
- The pH of a solution obtained by mixing 100mL of 0.1M HCI and 9.9mL of...
Text Solution
|
- What will be the resultant pH, when 200 mL of an aqueous solution of H...
Text Solution
|