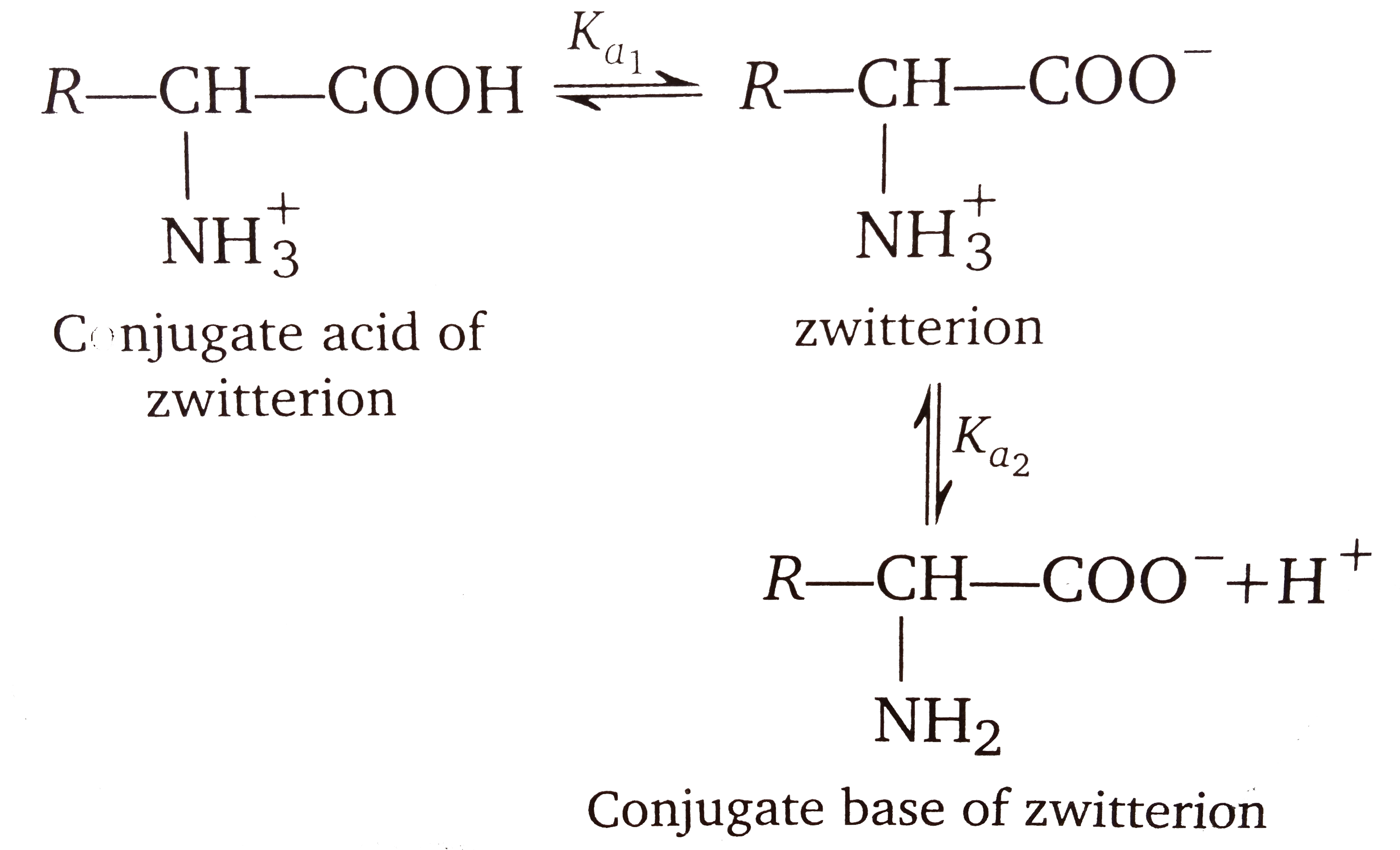
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
IONIC EQUILIBRIUM
P BAHADUR|Exercise Exercise3B|30 VideosIONIC EQUILIBRIUM
P BAHADUR|Exercise Exercise4|32 VideosIONIC EQUILIBRIUM
P BAHADUR|Exercise Exercise2|52 VideosGASEOUS STATE
P BAHADUR|Exercise Exercise -9|1 VideosMOLE AND EQUIVALENT CONCEPT
P BAHADUR|Exercise Exercise 9 Advanced numerical problems|61 Videos
Similar Questions
Explore conceptually related problems
P BAHADUR-IONIC EQUILIBRIUM-Exercise3A
- The correct order of increasing acidic strength is:
Text Solution
|
- Which of the following solution can be titrated with HCI as well as Na...
Text Solution
|
- The K(sp) of Mg(OH)(2) is 1xx10^(-12). 0.01M Mg^(2+) will precipitate ...
Text Solution
|
- H(2)S is passed through (CH(3)COO)(2) Zn and ZnCI(2) solutions separat...
Text Solution
|
- Select the incorrect statement about, R-underset(NH(2))underset(|)(C...
Text Solution
|
- At what pH will a 1xx10^(-4)M solution of an acid base indicator H In ...
Text Solution
|
- The correct relationship between the pH of isolomar solution of Na(2)O...
Text Solution
|
- At 25^(@)C K(b) for BOH=1.0xx10^(-12).0.01M solution of BOH has [OH^(-...
Text Solution
|
- Silver nitrate solution is gradually added to an aqueous conjuaining 0...
Text Solution
|
- Three sparingly soluble salts M(2)X, MX and MX(3) have the same solubi...
Text Solution
|
- In which of the following combinations, is buffer action expected ? ...
Text Solution
|
- Which of the following pairs consitutes buffer?
Text Solution
|
- The hydrogen ion concentration of a 10^(-8) M HCl aqueous soultion at ...
Text Solution
|
- The hydrolysis constant for the reaction, H(2)PO(4)^(-)+H(2)hArrH(3)P...
Text Solution
|
- pH of 0.1M Na(2)HPO(4) and 0.2M NaH(2)PO(4) are respectively: (pK(a)"f...
Text Solution
|
- C(2)H(5)ONa acts as ……. In C(2)H(5)OH.
Text Solution
|
- The degree of dissociation of C(6)H(5)COOH is influenced by :
Text Solution
|
- The pH of which compound in aqueous solution depends on its concentrat...
Text Solution
|
- The pH of the mixture when 50 mL each of 0.4M and 0.1M of HCI and NaC...
Text Solution
|
- n moles of N(2)O(5(s)) at pressure P^(@) at 300 K are taken in a clos...
Text Solution
|