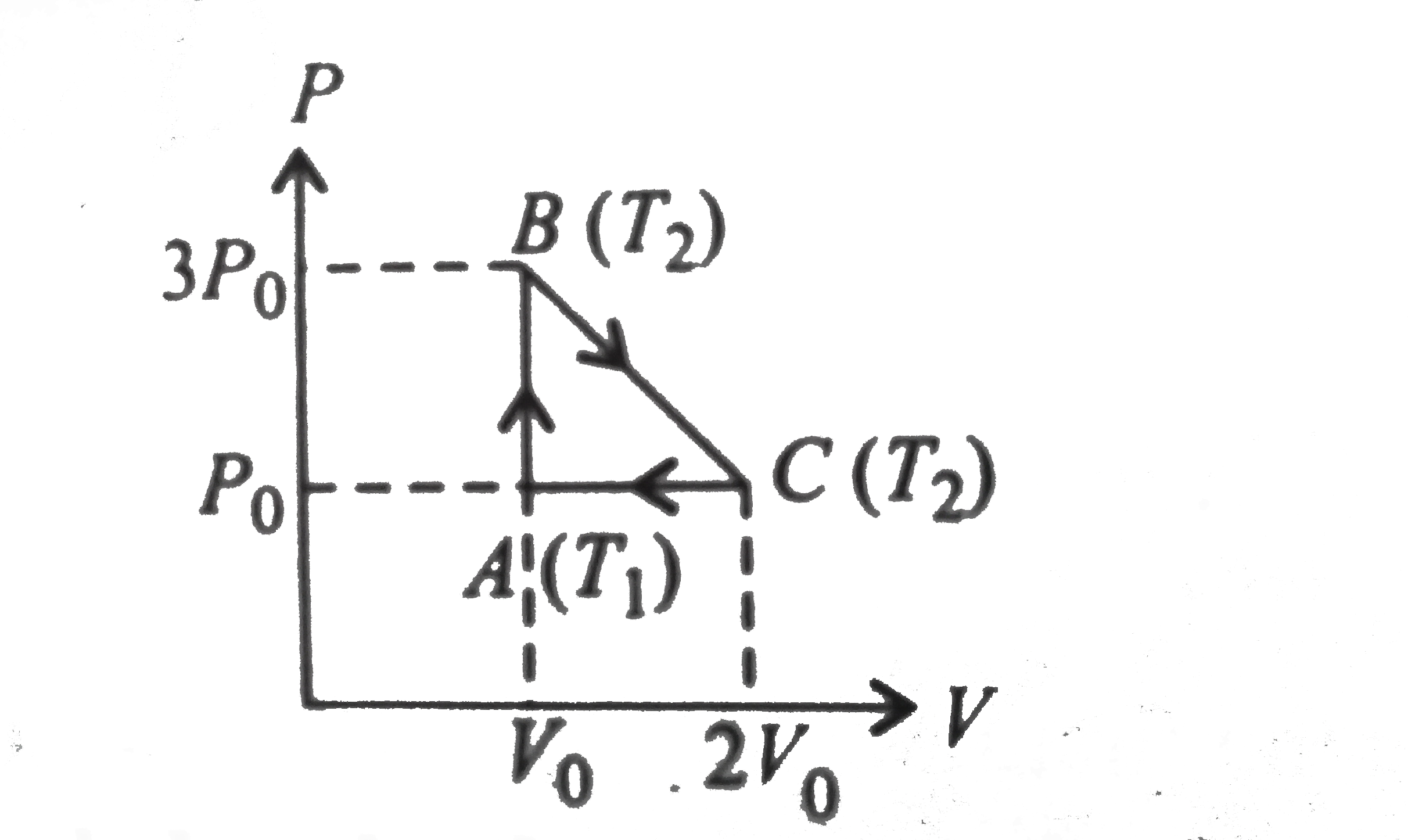Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
P BAHADUR-THERMODYNAMICS-Exercise
- A sample of argon gas at 1atm pressure and 27^@C expands reversibly an...
Text Solution
|
- One mole of an ideal gas is heated at constant pressure from 0^(@)C to...
Text Solution
|
- One mole of an ideal mono-atomic gas is taken round cyclic process ABC...
Text Solution
|
- Derive a relation showing reversible work of expansion from volume V1 ...
Text Solution
|
- What work is to be done on 2 mole of a perfect gas at 27^@C if it is c...
Text Solution
|
- Calculate the maximum work done in expanding 16g of oxygen at 300K occ...
Text Solution
|
- Calculateq, W, DeltaU and DeltaH for the isothermal reversible expansi...
Text Solution
|
- Calculate the change of entropy, Delta(r)S^(Theta) at 298K for the rea...
Text Solution
|
- The enthalpy of vaporisation of liquid diethly ether -(C2H5)2O, is 26....
Text Solution
|
- Ethanol boils at 78.4^@C and the enthalpy of vaporisation of ethanol i...
Text Solution
|
- Calculate the entropy change for the conservation of following: (a) 1g...
Text Solution
|
- Given S^@ for C("Graphite"), H(2(g)) and CH(4(g)) are 5.70, 130.7 and ...
Text Solution
|
- In a fuel cell, methanol if used as fuel and oxygen gas is used as an ...
Text Solution
|
- For the water gas reaction: C(s) +H(2)O(g) hArr CO(g) +H(2)(g) the...
Text Solution
|
- The standard Gibbs free energies for the reaction at 1773K are given b...
Text Solution
|
- Predict whether it is possible or not to reduce magnesium oxide using ...
Text Solution
|
- Calculate DeltaG^(Theta) for the conversion of oxygen to ozone, ((3)/(...
Text Solution
|
- Calculate the equilibrium constant Kp for the reaction given below if ...
Text Solution
|
- Calculate the value of DeltaG at 700K for the reaction, nX to mB. Give...
Text Solution
|
- Calculated the Gibbs energy change on dissolving one mole of sodium ch...
Text Solution
|