Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
P BAHADUR-THERMODYNAMICS-Exercise
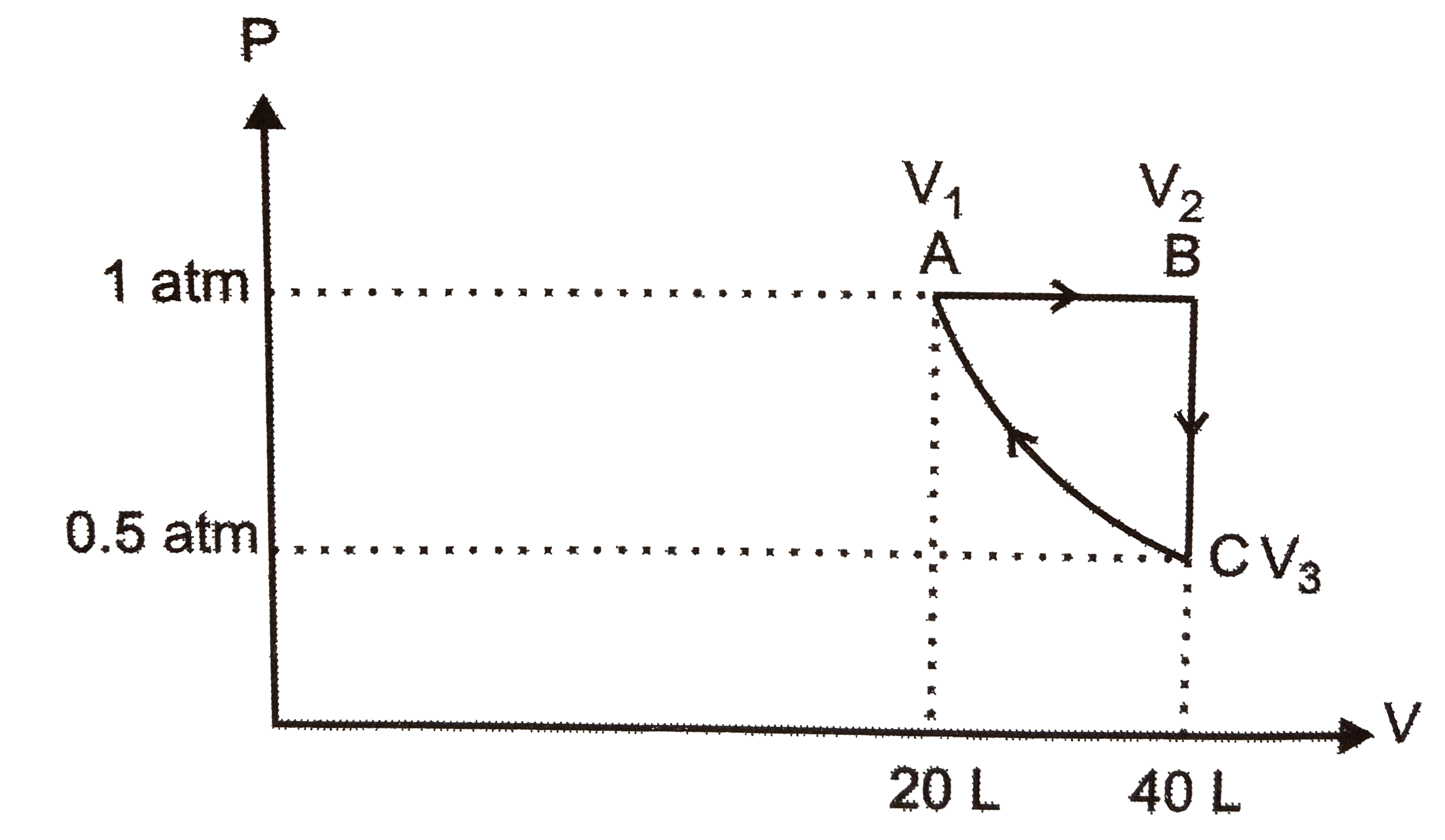
- A sample of argon gas at 1atm pressure and 27^(@)C expands reversibly ...
Text Solution
|
- 1mol of an ideal gas undergoes reversible isothermal expansion form an...
Text Solution
|
- Two moles of a perfect gas undergo the following process: (a) A revers...
Text Solution
|
- A gas expands from 3 dm^(3) to 5dm^(3) anainst a constant pressure of ...
Text Solution
|
- Show that the reaction CO(g) +(1//2)O(2)(g) rarr CO(2)(g) at 300K ...
Text Solution
|
- 100mL of a liquid is contained in an insulated container at a pressure...
Text Solution
|
- In which of the following cases entropy decreases?
Text Solution
|
- In a reaction DeltaG and DeltaH both are positive. The reaction would ...
Text Solution
|
- Temperature of 1mol of a gas is increased by 1^(@) at constant pressur...
Text Solution
|
- When a gas is subjected to adiabatic expansion, it gets cooled due to ...
Text Solution
|
- During isothermal expansion of an ideal gas, its:
Text Solution
|
- Change in entropy is negative for
Text Solution
|
- The DeltaG in the process of melting of ice at -15^@C is :
Text Solution
|
- A boiled egg show a//an…… in entropy.
Text Solution
|
- For the adiabatic expansion of an ideal gas:
Text Solution
|
- The occurrence of a reaction of impossible if
Text Solution
|
- Which statements is correct?
Text Solution
|
- An ideal gas undergoing expansion in vacuum shows:
Text Solution
|
- Human body is an example of:
Text Solution
|
- 1 mole of gas occupying 3 litre volume is expanded against a constant ...
Text Solution
|