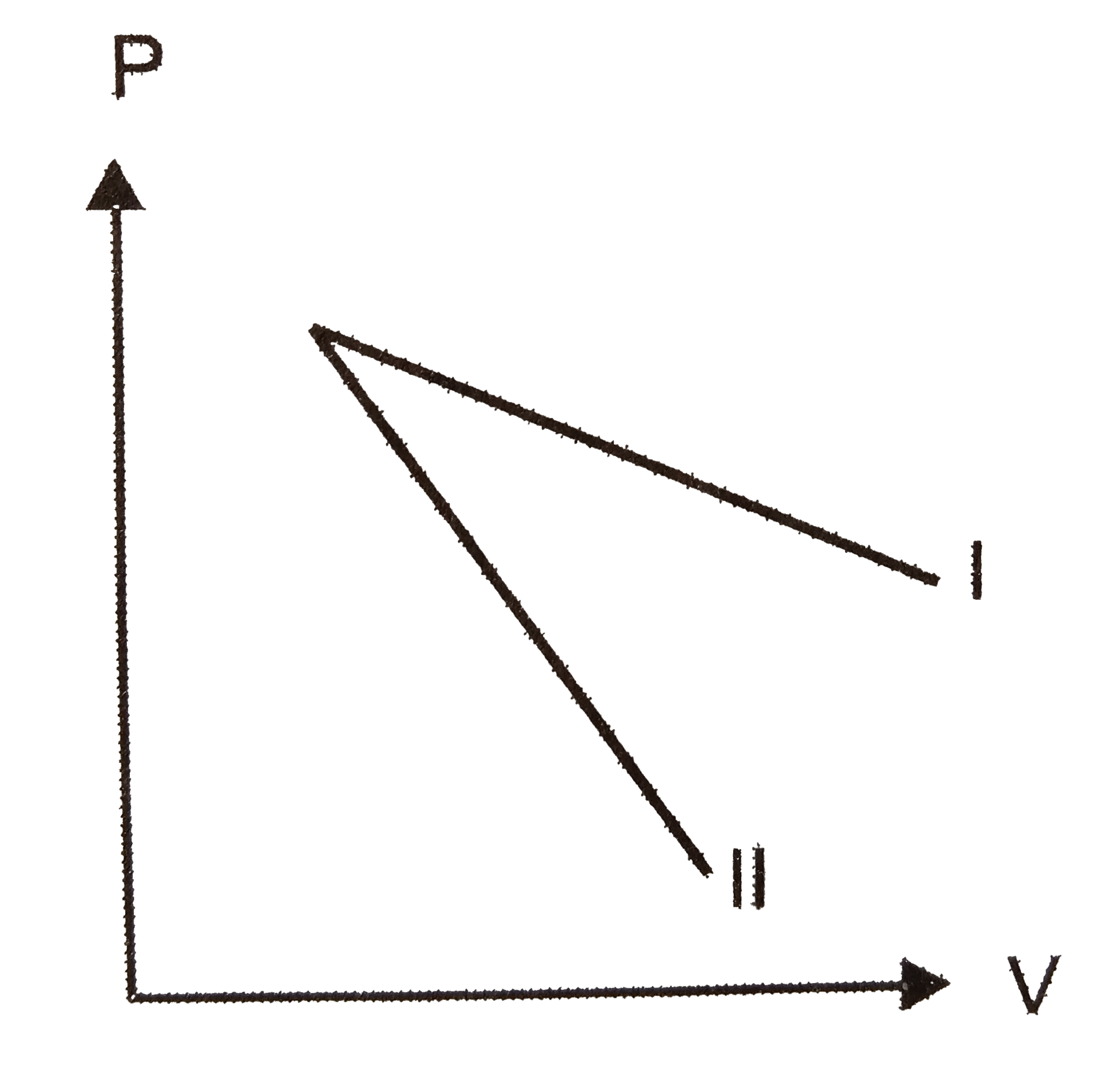
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
P BAHADUR-THERMODYNAMICS-Exercise
- A monoatomic gas is suddenly compressed to 1//8 of its original volume...
Text Solution
|
- The enthalpy and entropy change for the reaction, Br(2)(l)+Cl(2)(g)r...
Text Solution
|
- P-V plots for two gases during adiabatic expansion are shown in figure...
Text Solution
|
- For an adiabatic expansion of a perfect gas dP//P is equal to :
Text Solution
|
- The poisson's ratio for O(2) is 1.4. Which of the following are correc...
Text Solution
|
- Which species possesses negative value of specific heat?
Text Solution
|
- In which process net work done is zero ?
Text Solution
|
- The net work done through a series of changes reported in figure for a...
Text Solution
|
- Specific heat of a substance at m.pt. or b.pt. for isothermal process ...
Text Solution
|
- If for the reaction, 2Mg((s)) + O(2(g)) to 2MgO((s)), at 27^@C Delt...
Text Solution
|
- The ratio of slopes of P-V plots for reversible adiabatic process and ...
Text Solution
|
- 300K a gas (gamma = 5//3) is compressed suddenly so that its pressure ...
Text Solution
|
- Select the incorrect statements for the equillibrium under standard co...
Text Solution
|
- Heat of neutralisation of strong acid and strong base under 1 atm and ...
Text Solution
|
- The relation between the volume and temperature of a sample of water i...
Text Solution
|
- The work done in changing the state from (P0, V0) to (P1, V1) in the f...
Text Solution
|
- If the pressure on density of a diatomic gas (gamma=7/5) in adiabatic ...
Text Solution
|
- One box containing 1 mole of He is 7//3 T0 and other box containing 1 ...
Text Solution
|
- The entropy change in melting 1g of ice at 0^@C. (Latent heat of ice i...
Text Solution
|
- 50 students sitting in the room of 5 xx 10 xx 3m^(3) dimensions. The a...
Text Solution
|