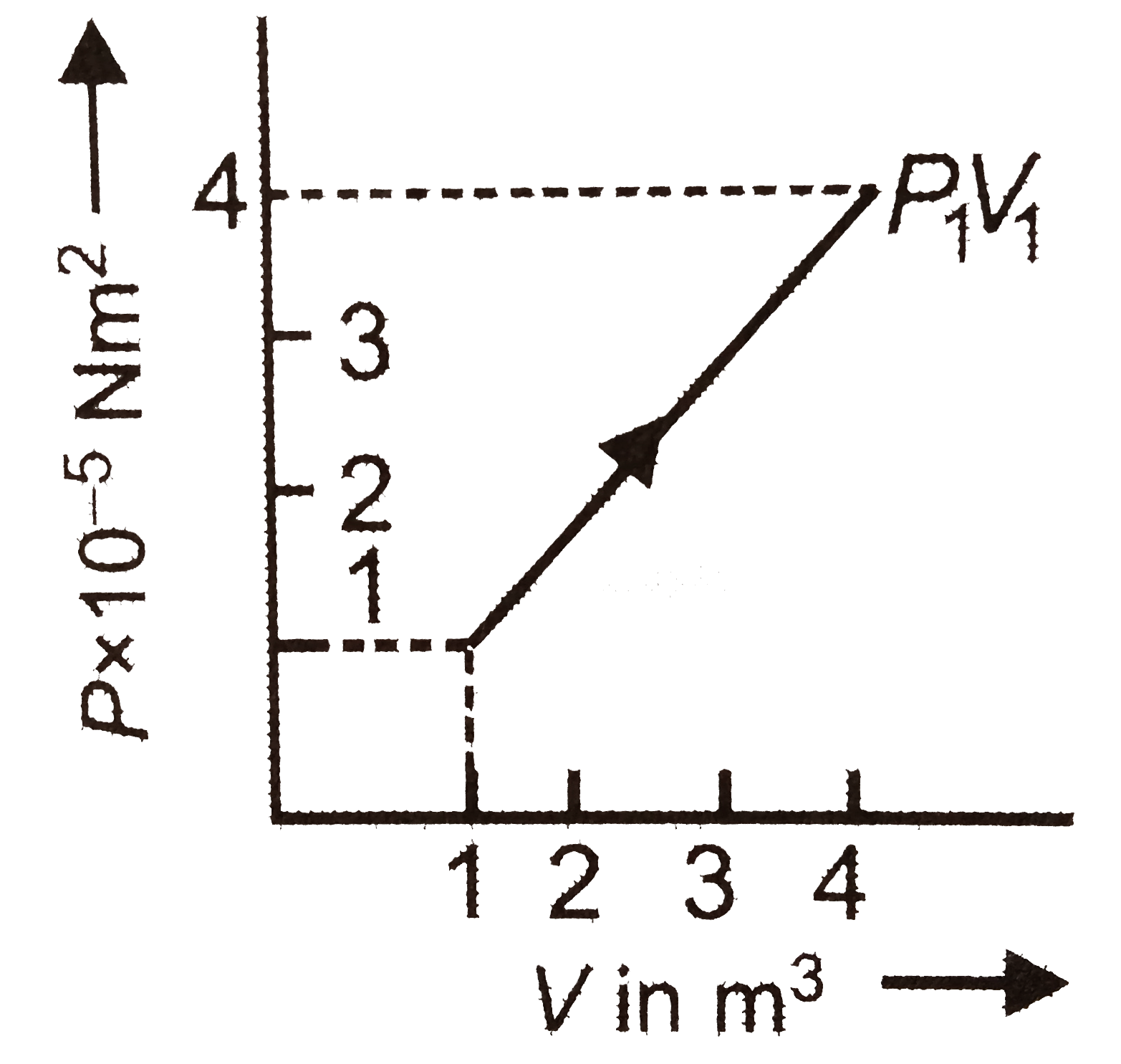
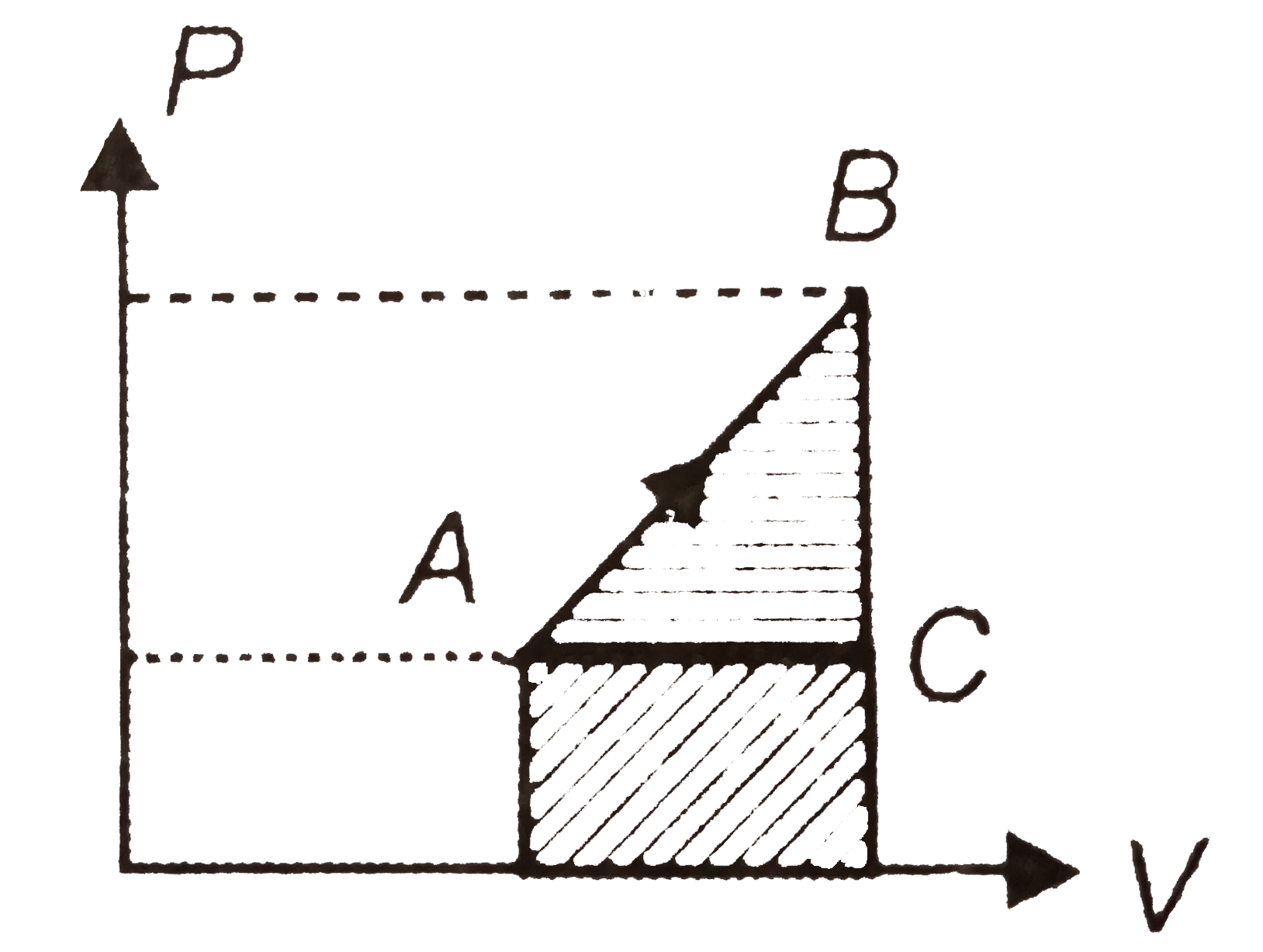
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
P BAHADUR-THERMODYNAMICS-Exercise
- Heat of neutralisation of strong acid and strong base under 1 atm and ...
Text Solution
|
- The relation between the volume and temperature of a sample of water i...
Text Solution
|
- The work done in changing the state from (P0, V0) to (P1, V1) in the f...
Text Solution
|
- If the pressure on density of a diatomic gas (gamma=7/5) in adiabatic ...
Text Solution
|
- One box containing 1 mole of He is 7//3 T0 and other box containing 1 ...
Text Solution
|
- The entropy change in melting 1g of ice at 0^@C. (Latent heat of ice i...
Text Solution
|
- 50 students sitting in the room of 5 xx 10 xx 3m^(3) dimensions. The a...
Text Solution
|
- DeltaH and DeltaS for the system H2O((l))hArr H2O((g)) at 1atm are 40....
Text Solution
|
- Two moles of ideal gas at 27^(@)C temperature is expanded reversibly f...
Text Solution
|
- Standard enthalpy and standard entropy change for the oxidation of NH(...
Text Solution
|
- One mole of an ideal gas at 27^@C expanded isothermally from an initia...
Text Solution
|
- The molar heat capacity of water at constant pressure P , is 75JK^(-1)...
Text Solution
|
- An ideal gas heat engine operates in Carnot cycle between 227^@C and 1...
Text Solution
|
- Which of the following graphs given below show (s) adiabatic process?
Text Solution
|
- In an adiabatic expansion of air (assume it a mixture of N2 and O2), t...
Text Solution
|
- A monoatomic gas is suddenly compressed to 1//8 of its original volume...
Text Solution
|
- The process of evaporation of a liquid is accompanied by :
Text Solution
|
- All natural processes proceed spontaneously in a direction which :
Text Solution
|
- Which of the following is (are) correct an adiabatic process?
Text Solution
|
- If q is the amount of heat absorbed by the system and W the amount of ...
Text Solution
|