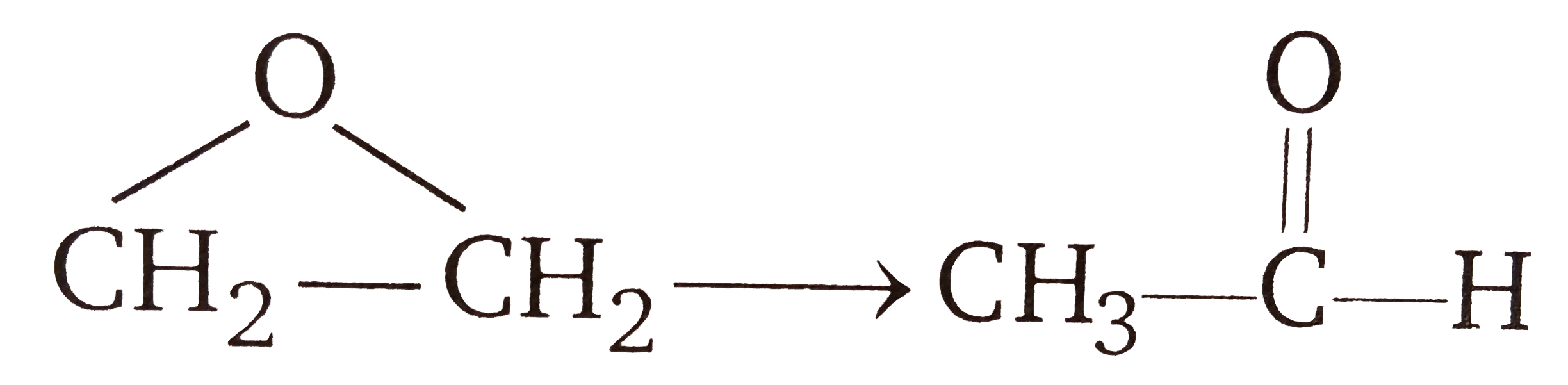A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
P BAHADUR-THERMODYNAMICS-Exercise
- The open system (s) is (are) which :
Text Solution
|
- Which of the following statements is/are correct?
Text Solution
|
- Given: for ethylene oxide DeltaH^@f and DeltaS^@ are -51kJmol^(-1), 24...
Text Solution
|
- The reversible expansion of an ideal gas under adiabatic and isotherma...
Text Solution
|
- For an ideal gas, consider only P-V work in going from an initial stat...
Text Solution
|
- An endotthermic reaction is non-spontaneous at freezing point of water...
Text Solution
|
- A heat engine absorbs heat Q(1) at temperature T(1) and Q(2) at temper...
Text Solution
|
- In an irreversible process taking place at constant T and P and in whi...
Text Solution
|
- The internal energy change when a system goes fromk state A to B is 40...
Text Solution
|
- The correct relationship between free energy change in a reaction and ...
Text Solution
|
- An ideal gas expands from 1xx10^(-3)m^(3) to 1xx10^(-2)m^(3) at 300K a...
Text Solution
|
- For a spontaneous reaction, DeltaG , equilibrium constant (K) and E(ce...
Text Solution
|
- An ideal gas is allowed to expand both reversibly and irreversibly in ...
Text Solution
|
- In conversion of lime-stone to lime, CaCO(3(s)) to CaO((s)) + CO(2(g...
Text Solution
|
- Standard entropy of X(2) , Y(2) and XY(3) are 60, 40 and 50JK^(-1)mol...
Text Solution
|
- Which one of the following statement is false?
Text Solution
|
- In thermodynamics, a process is called reversible when
Text Solution
|
- One mole of a non-ideal gas undergoes a change of state (2.0atm,3.0L,9...
Text Solution
|
- Two moles of an ideal gas expanded isothermally and reversibly from 1L...
Text Solution
|
- The enthalpy of vaporisation of a liquid is 30 kJ mol^(-1) and entropy...
Text Solution
|