A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
P BAHADUR-THERMODYNAMICS-Exercise
- The first law of thermodynamics was given as q=DeltaU+(-w), where q is...
Text Solution
|
- The first law of thermodynamics was given as q=DeltaU+(-w), where q is...
Text Solution
|
- The first law of thermodynamics was given as q=DeltaU+(-w), where q is...
Text Solution
|
- Work done by the system in isothermal reversible process is w(rev.)= -...
Text Solution
|
- Work done by the system in isothermal reversible process is w(rev.)= -...
Text Solution
|
- Work done by the system in isothermal reversible process is w(rev.)= -...
Text Solution
|
- Work done by the system in isothermal reversible process is w(rev.)= -...
Text Solution
|
- A flask of 1L having NH(3)(g) at 2.0atm and 200K is connected with the...
Text Solution
|
- A flask of 1L having NH(3)(g) at 2.0atm and 200K is connected with the...
Text Solution
|
- A flask of 1L having NH(3)(g) at 2.0atm and 200K is connected with the...
Text Solution
|
- Assertion: Work and internal energy are not state functions. Reason:...
Text Solution
|
- Statement: Internal energy of a system is an extensive property. Exp...
Text Solution
|
- Statement: The change in internal energy and change in heat enthalpy d...
Text Solution
|
- Statement: Both work and heat are manifested by an effect in the surro...
Text Solution
|
- Assertion: The zeroth law of thermodynamics was know before the first ...
Text Solution
|
- Statement: The entropies of CO,NO,N(2)O,Cl(2(s)) are not zero at absol...
Text Solution
|
- Assertion:Phase transition involves change in internal energy only. ...
Text Solution
|
- Assertion:The change in entropy during melting of ice is negligible in...
Text Solution
|
- Assertion:The SIunit of entropy isJK^(-1)mol^(-1) Reason:DeltaS=(q(r...
Text Solution
|
- Statement: The thermodynamics functions which determines the spontanei...
Text Solution
|
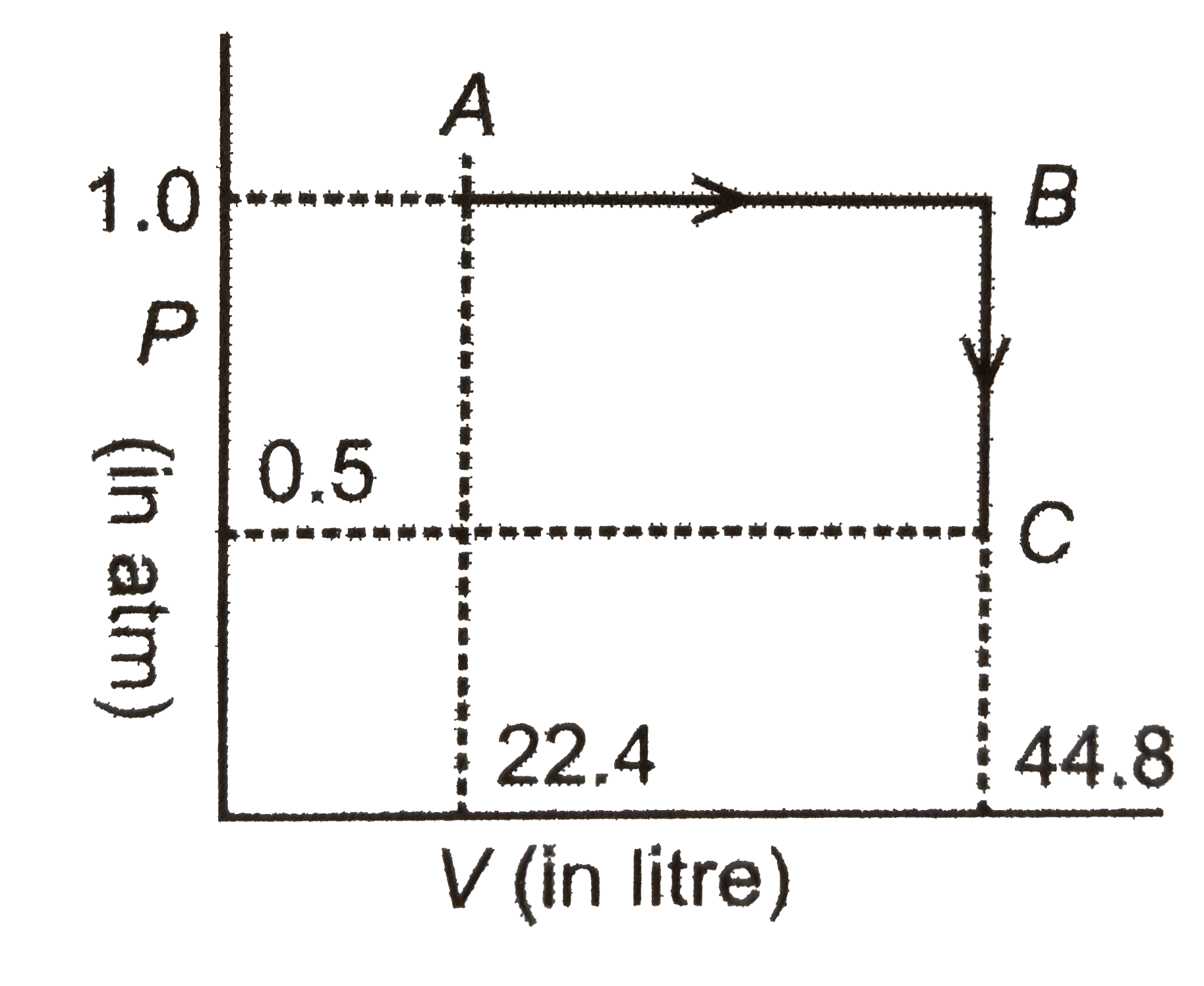 select the correct statements:
select the correct statements: