Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
P BAHADUR-THERMODYNAMICS-Exercise
- One mole of a monoatomic ideal gas is heated at constant pressure from...
Text Solution
|
- Calculate the final temperature of a sample of CO(2) gas(16g) that is ...
Text Solution
|
- One mole of a perfect gas is put through a cycle consisting of the fol...
Text Solution
|
- 1g of water changes from liquid to vapour phase at constant pressure o...
Text Solution
|
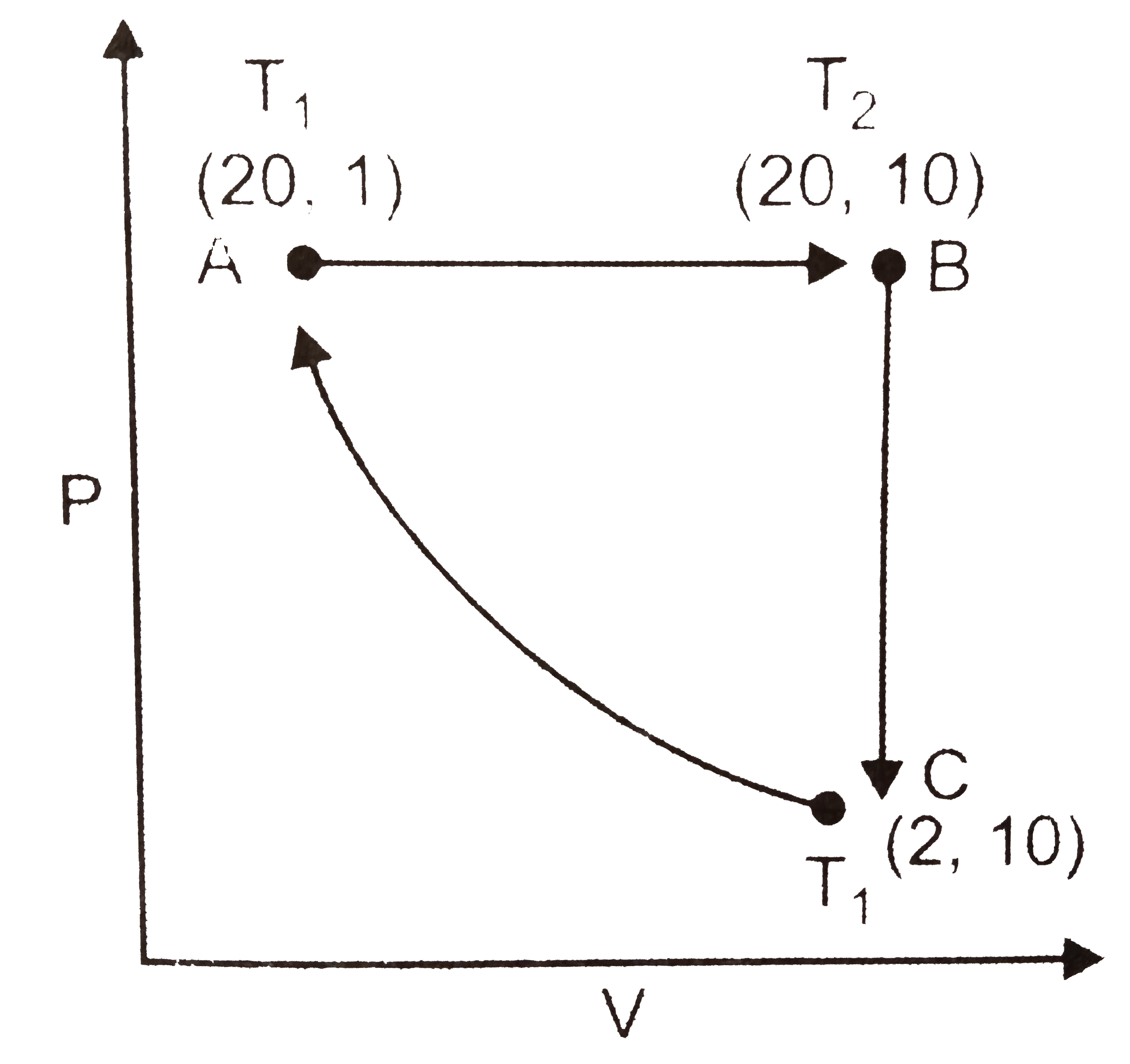
- The given figure shown a change of state A to state C by two paths ABC...
Text Solution
|
- A monoatomic ideal gas of two moles is taken through a cyclic process ...
Text Solution
|
- Two moles of helium gas (r=5//3) are initially at a temperature of 27^...
Text Solution
|
- An ideal gas has a specific heat at constant pressure C(p)=5/2R. The g...
Text Solution
|
- A strip of magnesium of mass 15 g is droped into an opean beaker of di...
Text Solution
|
- Calculate the work done when 56g of iron reacts with hydrochloric acid...
Text Solution
|
- The internal energy change in the conversion of 1.0 mole of the calcit...
Text Solution
|
- Calculate the work done when a system raises a colume of water of radi...
Text Solution
|
- A bulb of 100 watt is switched on in a room of dimensions 5xx4xx3 m^(3...
Text Solution
|
- For a reaction M(2)O((g))to2M((s))+1/2O(2(g)), DeltaH=30 k J mol^(-1...
Text Solution
|
- Consider a class room of dimesions 5 xx 10 xx 3m^(3) at temperature 20...
Text Solution
|
- An athelete in a gymansium room lifts a 50 kg mass through a vertical ...
Text Solution
|
- An aeroplane weighing 63,000 kg flies up from sea level to a height of...
Text Solution
|
- Titanium metal is extensively used in aerospace industry because the m...
Text Solution
|
- A lead bullet weighing 18.0g and travelling at 500 m//s is embedded in...
Text Solution
|
- The standard enthalpy and entropy changes for the reaction in equilibr...
Text Solution
|