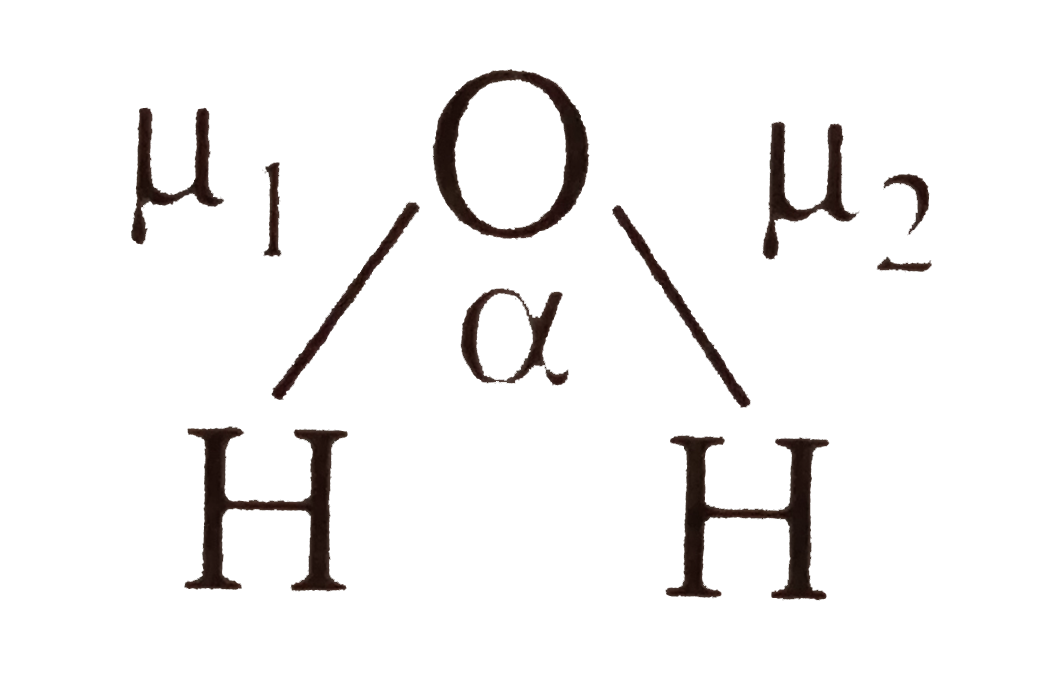Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
P BAHADUR-CHEMICAL BONDING-Exercise 7
- The experimental dipole moment of water molecule is 1.84D . Calculat...
Text Solution
|
- Statement : IE1 for is maximum and EA1 for Cl is more than EA1 of (F)...
Text Solution
|
- Statement : If difference of electronegtivity beteen two atoms is zero...
Text Solution
|
- Statement : p-dimethoxy benzene is polar molecule . Explanation : T...
Text Solution
|
- Statement : The lattice energy of silver halids is AgF gt AgCl gt AgB...
Text Solution
|
- Statement : The molecule cis-1-chloropropene is nore polar than trans...
Text Solution
|
- Statemet : IF7 is super-ociet molecule . Explanation : central atom ...
Text Solution
|
- Statement : FeCl2 is more covalent than FeCl3 Explanation : Higher i...
Text Solution
|
- Statement : MO configuration of CO is : sigma 1s^2 , sigma^**1s^2 , ...
Text Solution
|
- Statement : The dipole moment of NH3 is less than NF3 . Explanation...
Text Solution
|
- Statement : The bond energy of P-Cl bond in PCl3 and PCl5 different . ...
Text Solution
|
- Statement : SF4 has lone pair of electron at equatorial position in pr...
Text Solution
|