A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
P BAHADUR-CHEMICAL BONDING-Exercise2A
- Which bond angel theta would result in the maximum dipole moment for...
Text Solution
|
- Dipole moment is highest for:
Text Solution
|
- The molecules having dipole moment are :
Text Solution
|
- Bond angle between two hybrid orbitals is 106^@ Hybride charcter in or...
Text Solution
|
- In the cyanide ion, the formal negative charge is on :
Text Solution
|
- Which statement is correct about HCHO ?
Text Solution
|
- Which of the following halides is not oxidised by M no2 ?
Text Solution
|
- The enolic form of acetone contains:
Text Solution
|
- Amongst LiCl, RbCl, BeCl2 and MgCl2, the compounds whith the greatrest...
Text Solution
|
- The total number of valence electrons in 4. 2g of N3^- ion are :
Text Solution
|
- In piperdine . N -H, N atom has hybridisation :
Text Solution
|
- One among the following is the incrorrect order of increasing ionisati...
Text Solution
|
- CuSO4. 5H2 O is represented as :
Text Solution
|
- The correct order of increasing electropositive character among Cu . F...
Text Solution
|
- Which shows a changes in the type of hybridisation when :
Text Solution
|
- When temperature is lowered NO2 dimerises . It is accompanised by :
Text Solution
|
- The dipole moment of NF3 is very much less compatred to that of NH3 be...
Text Solution
|
- In HCHO, there are X no-bonding electron pairs Y sigama -bonds and Z ...
Text Solution
|
- For compounds , A : Tetracuanoethern B : Carbon dioxide C: Ben...
Text Solution
|
- Hypervalent compound is :
Text Solution
|
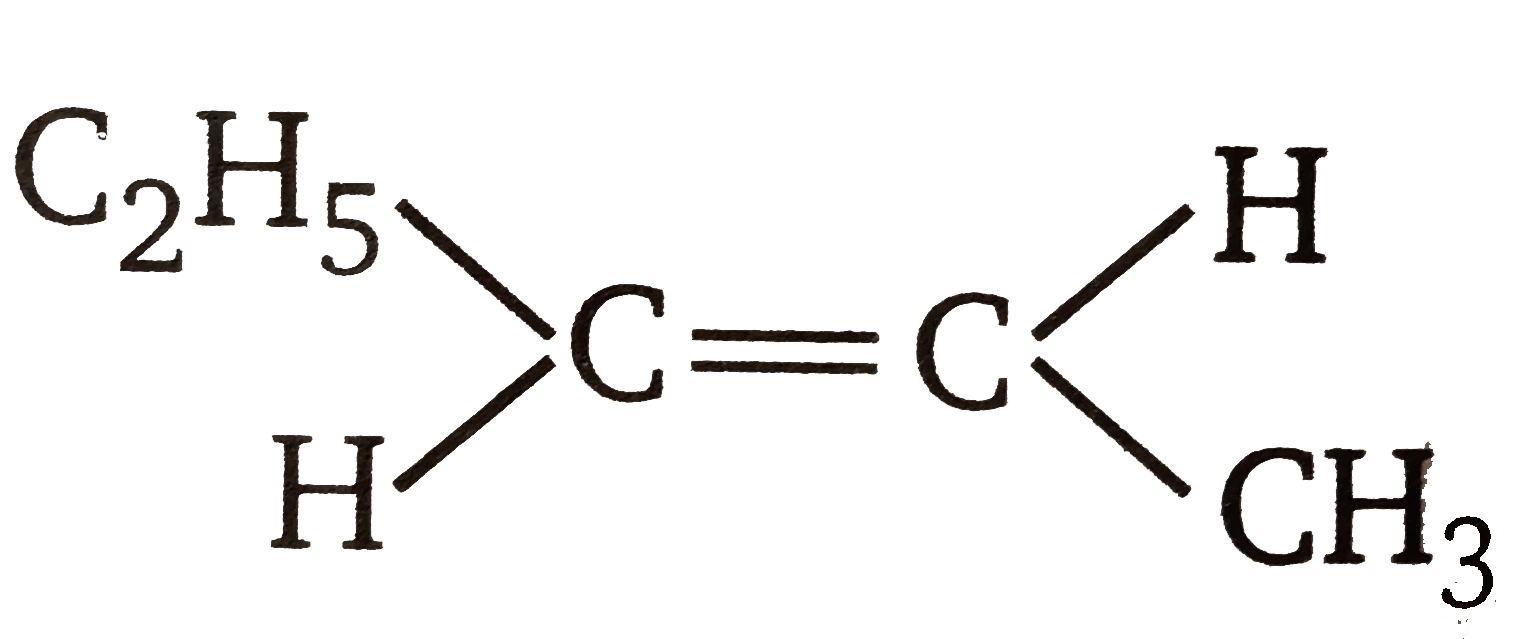 .
.