A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
P BAHADUR-CHEMICAL BONDING-Exercise2A
- Which shows a changes in the type of hybridisation when :
Text Solution
|
- When temperature is lowered NO2 dimerises . It is accompanised by :
Text Solution
|
- The dipole moment of NF3 is very much less compatred to that of NH3 be...
Text Solution
|
- In HCHO, there are X no-bonding electron pairs Y sigama -bonds and Z ...
Text Solution
|
- For compounds , A : Tetracuanoethern B : Carbon dioxide C: Ben...
Text Solution
|
- Hypervalent compound is :
Text Solution
|
- Which set contains par of elements that do not belong to same froup bu...
Text Solution
|
- An element of p-block in which last electron enters into s-orbital of ...
Text Solution
|
- Covelant radius of Liis 123 pm .The crystal radius of Li will be:
Text Solution
|
- A molecule which cannot exist theoretcally is :
Text Solution
|
- Which are true statements among the following ?
Text Solution
|
- The structure of IF4 can be best desribed by :
Text Solution
|
- The solublity of KCl is relatively more in :
Text Solution
|
- Which of the following possess lowest bond energy ?
Text Solution
|
- Molecular size of ICI and Br2 is nearly same but b.pt. of ICI is abou...
Text Solution
|
- The pair of species having identical shape is :
Text Solution
|
- The molecule having three fold axid of symmetry is :
Text Solution
|
- Which of the following phenomenon will occure when tow atoms of an ele...
Text Solution
|
- Structrue of ICO2^- is :
Text Solution
|
- The most suitable method of separation of a mixture of ortho and para ...
Text Solution
|
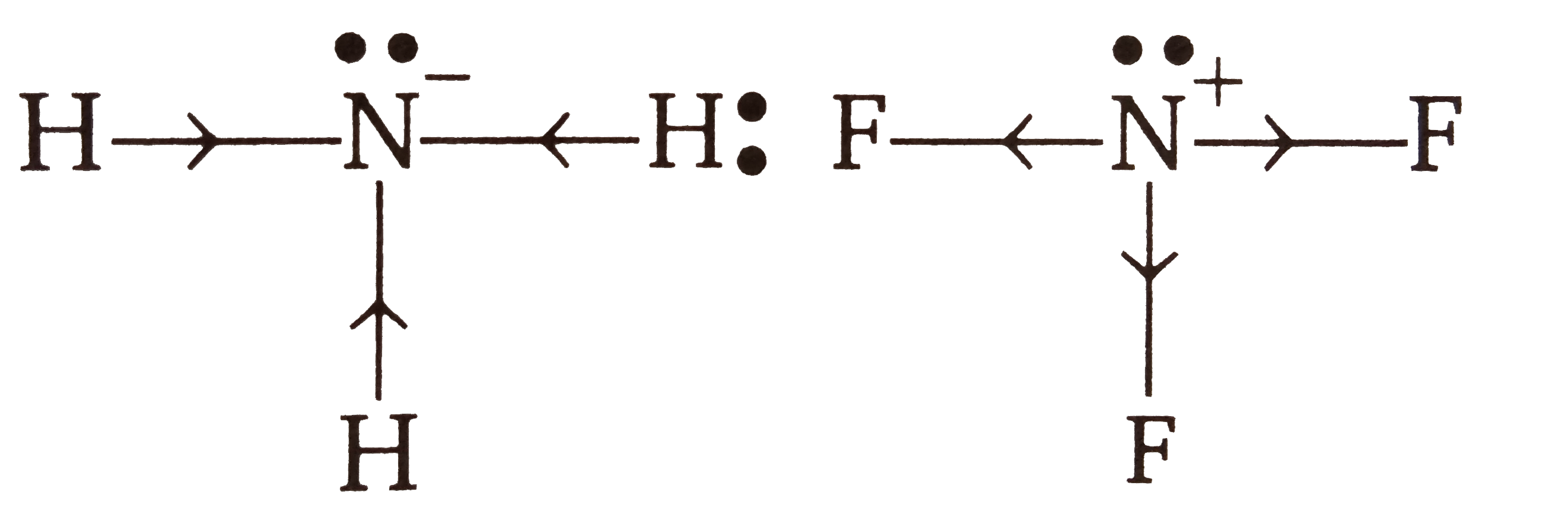 .
.