A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
P BAHADUR-CHEMICAL BONDING-Exercise2A
- Which of the following molecule froms linear polymeric structure due t...
Text Solution
|
- The correct roder o fincreasing C-O bond lengths in CO, CO3^(2-) and ...
Text Solution
|
- Among the following which has resonating structure ?
Text Solution
|
- For which crystalline substance does the solubility in water increase ...
Text Solution
|
- Which among the following is true ?
Text Solution
|
- During the formation of a molecular orbital from atomic orbitral , th...
Text Solution
|
- The shapes of PCl4^+, PCl4^- and AsCl4 are respectively :
Text Solution
|
- Which of the following paires of elements for oxideas of polyanions a...
Text Solution
|
- N -O -N bond andle is maximum in :
Text Solution
|
- Which is not a permissible resonating structure ?
Text Solution
|
- Which pair represents canonical from ?
Text Solution
|
- Which of the following would have permanent dipple moment ?
Text Solution
|
- The correct order of increasing covalent charactre is :
Text Solution
|
- Which one of the following orders is not correct in accordance whith ...
Text Solution
|
- The electronegaivity difference between N and F is greater than that b...
Text Solution
|
- In which of the following moleucles are all the bonds not equal ?
Text Solution
|
- Which of the following is not a correct statement ?
Text Solution
|
- Which of the following species has a linear shape ?
Text Solution
|
- Which of the following is not isostructural with SiCkl4 ?
Text Solution
|
- The number of unpaired electrons in a parmamagnetic diatomic molecyle ...
Text Solution
|
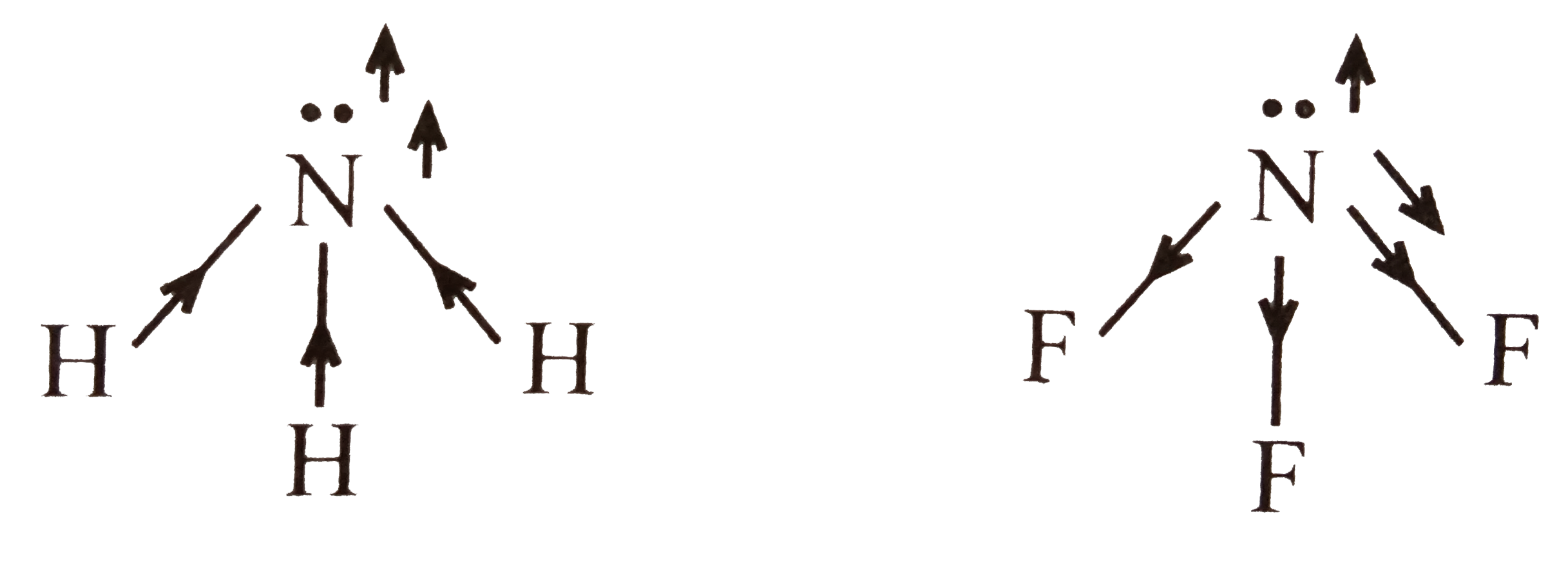 .
.