Text Solution
Verified by Experts
Topper's Solved these Questions
THERMODYNAMICS
PRADEEP|Exercise Short answer questions(NCERT)|6 VideosTHERMODYNAMICS
PRADEEP|Exercise Long answer questions(NCERT)|5 VideosTHERMODYNAMICS
PRADEEP|Exercise Advanced problems for competitions|10 VideosSYSTEMS OF PARTICLES AND ROTATIONAL MOTION
PRADEEP|Exercise Assertion- Reason Type questions|20 VideosWORK, ENERGY AND POWER
PRADEEP|Exercise Assertion-Reason Type Questions|24 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-THERMODYNAMICS-Very short answer questions(NCERT)
- Can a system be heated and its temperature remains constant?
Text Solution
|
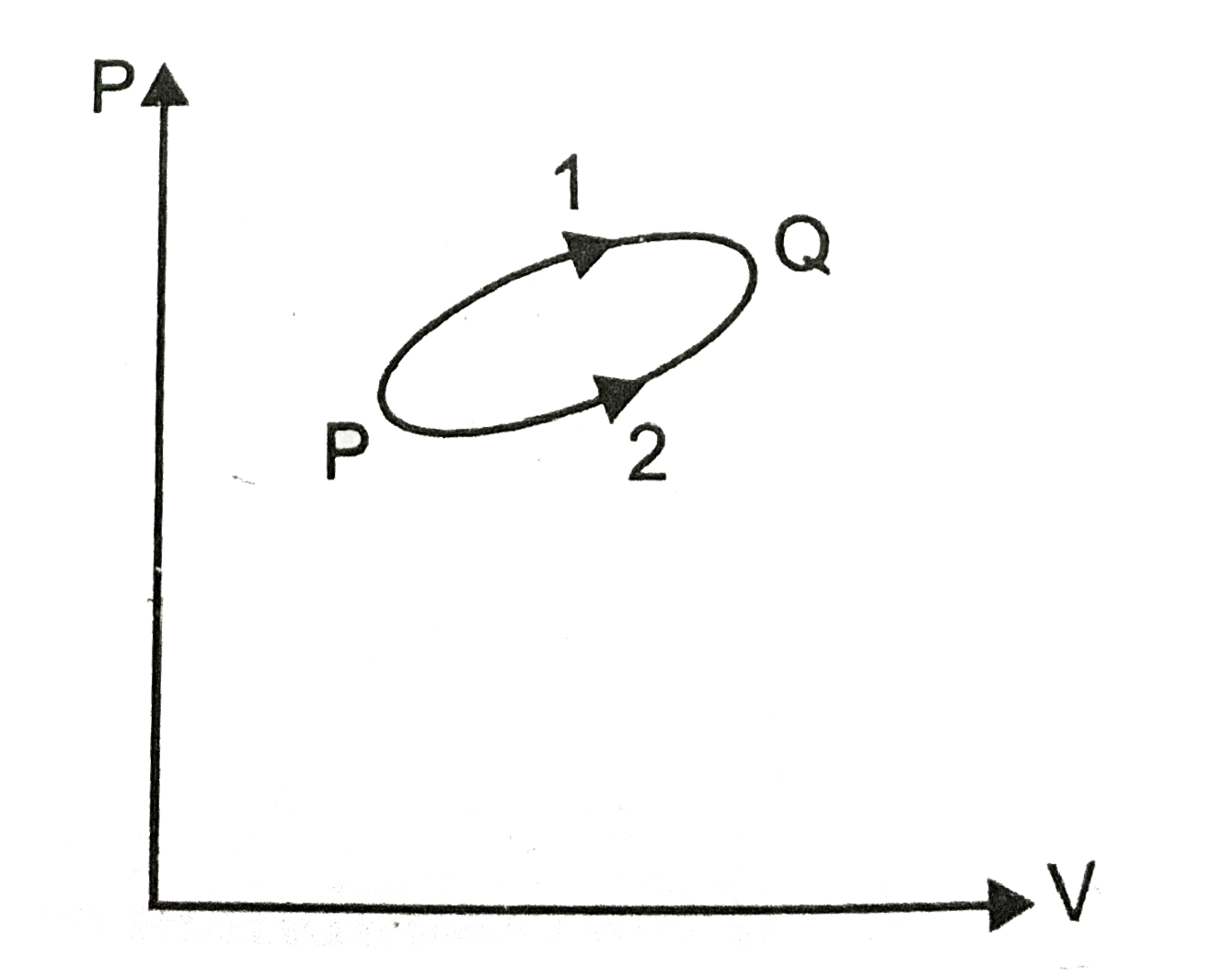
- A system goes from P to Q by two different paths in the P-V diagram as...
Text Solution
|
- On a hot summer day we want to cool our room by opening the refrigerat...
Text Solution
|
- Can we increase the temperature of a gas without supplying heat to it?
Text Solution
|
- Air pressure in a car tyre increases during driving. Explain.
Text Solution
|