Text Solution
Verified by Experts
Topper's Solved these Questions
THERMODYNAMICS
PRADEEP|Exercise Long answer questions(NCERT)|5 VideosTHERMODYNAMICS
PRADEEP|Exercise Higher order thinking skills|9 VideosTHERMODYNAMICS
PRADEEP|Exercise Very short answer questions(NCERT)|5 VideosSYSTEMS OF PARTICLES AND ROTATIONAL MOTION
PRADEEP|Exercise Assertion- Reason Type questions|20 VideosWORK, ENERGY AND POWER
PRADEEP|Exercise Assertion-Reason Type Questions|24 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-THERMODYNAMICS-Short answer questions(NCERT)
- Consider a Carnot's cycle operating betweenT(1)= 500K and T(2)= 300K p...
Text Solution
|
- A person of mass 60 Kg wants to lose 5 kg by going up and down a 10 m ...
Text Solution
|
- Consider a cycle tyre being filled with air by a pump. Let V be the vo...
Text Solution
|
- In a refrigerator, one removes heat from a lower temperature and depos...
Text Solution
|
- If the co-efficient of performance of a refrigerator is 5 and operates...
Text Solution
|
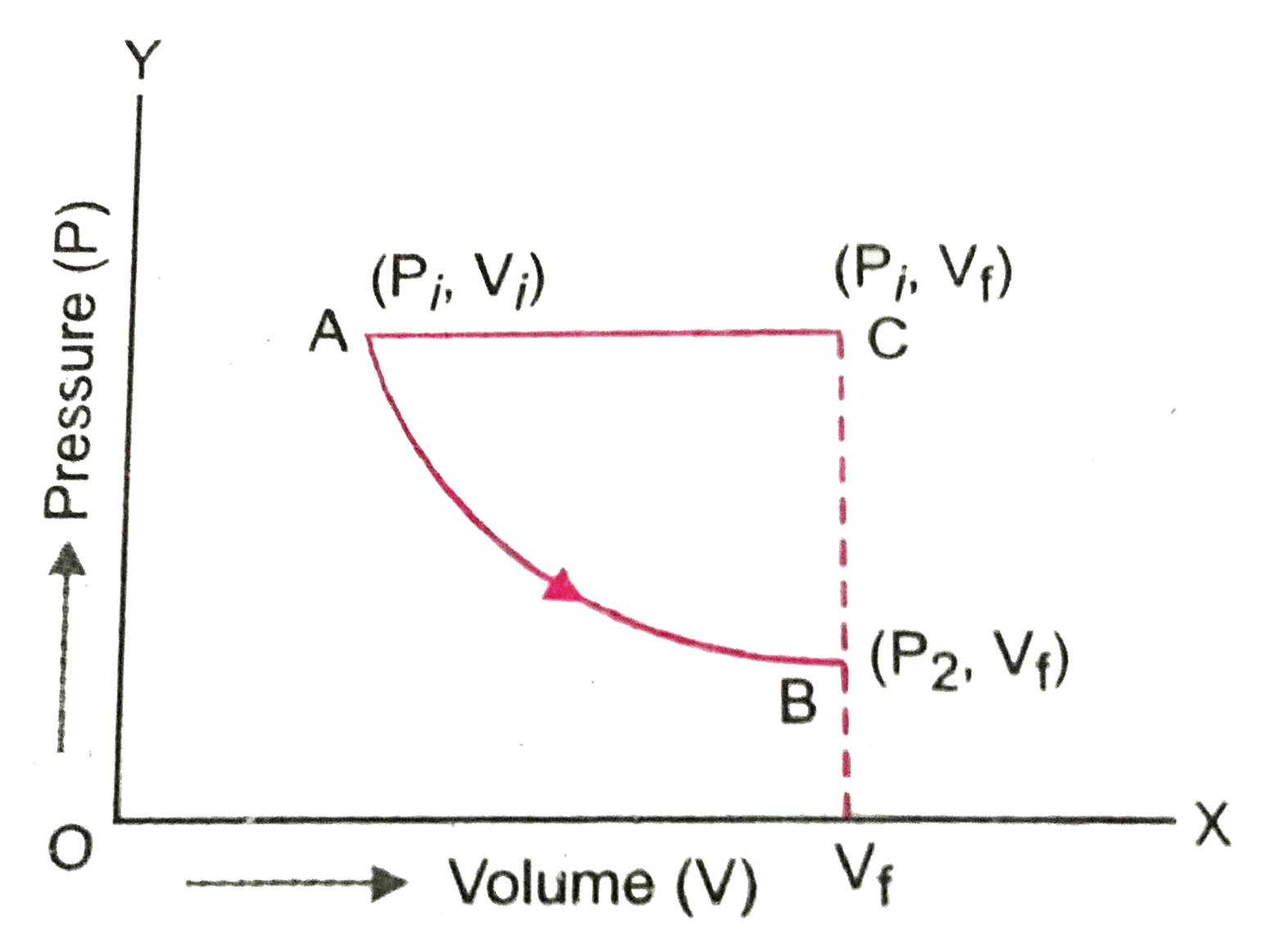
- The initial state of certain gas is (P(i), V(i), T(i)). It undergoes e...
Text Solution
|