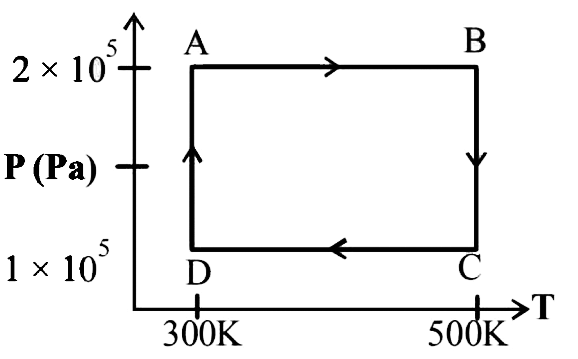
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THERMODYNAMICS
PRADEEP|Exercise Interger Type questions|11 VideosTHERMODYNAMICS
PRADEEP|Exercise Assertion- Reason Type Questions|19 VideosTHERMODYNAMICS
PRADEEP|Exercise Multiple choice questions (NCERT)|10 VideosSYSTEMS OF PARTICLES AND ROTATIONAL MOTION
PRADEEP|Exercise Assertion- Reason Type questions|20 VideosWORK, ENERGY AND POWER
PRADEEP|Exercise Assertion-Reason Type Questions|24 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-THERMODYNAMICS-Multiple choice questions.
- In the given (V-T) diagram, what is the relation between pressure P(1)...
Text Solution
|
- The P-V diagram of a gas undergoing a cyclic process ABCDA is shown in...
Text Solution
|
- Two moles of helium gas are taken over the cycle ABCDA, as shown in th...
Text Solution
|
- Two moles of helium gas are taken over the cycle ABCDA, as shown in th...
Text Solution
|
- Two moles of helium gas are taken over the cycle ABCDA, as shown in th...
Text Solution
|
- A monoatomic gas at pressure P(1) and volume V(1) is compressed adiaba...
Text Solution
|
- An ideal gas is made go to through a cyclic thermodynamics process in ...
Text Solution
|
- A thermally insulated vessel contains an ideal gas of molecular mass M...
Text Solution
|
- During an isothermal expansion, a confined ideal gas does -150 J of wo...
Text Solution
|
- When 1 kg of ice at 0^(@)C melts to water at 0^(@)C, the resulting cha...
Text Solution
|
- A mass of diatomic gas(gamma=1.4) at a pressure of 2 atomphere is comp...
Text Solution
|
- 5.6 liter of helium gas at STP is adiabatically compressed to 0.7 lite...
Text Solution
|
- Two moles of ideal helium gas are in a rubber balloon at 30^@C. The ba...
Text Solution
|
- Helium gas goes through a cycle ABCDA (consisting of two isochoric and...
Text Solution
|
- One mole of an ideal gas goes from an initial state A to final state B...
Text Solution
|
- An ideal gas goes from State A to state B via three different process ...
Text Solution
|
- During an adiabatic process, the pressure of a gas is found to be prop...
Text Solution
|
- The amount of heat energy required to raise the temperature of 1 g of ...
Text Solution
|
- A gas is taken through the cycle A rarrB rarr C rarr A, as shown in fi...
Text Solution
|
- Steam at 100^(@)C is passed into 20 g of water at 10^(@)C when water a...
Text Solution
|