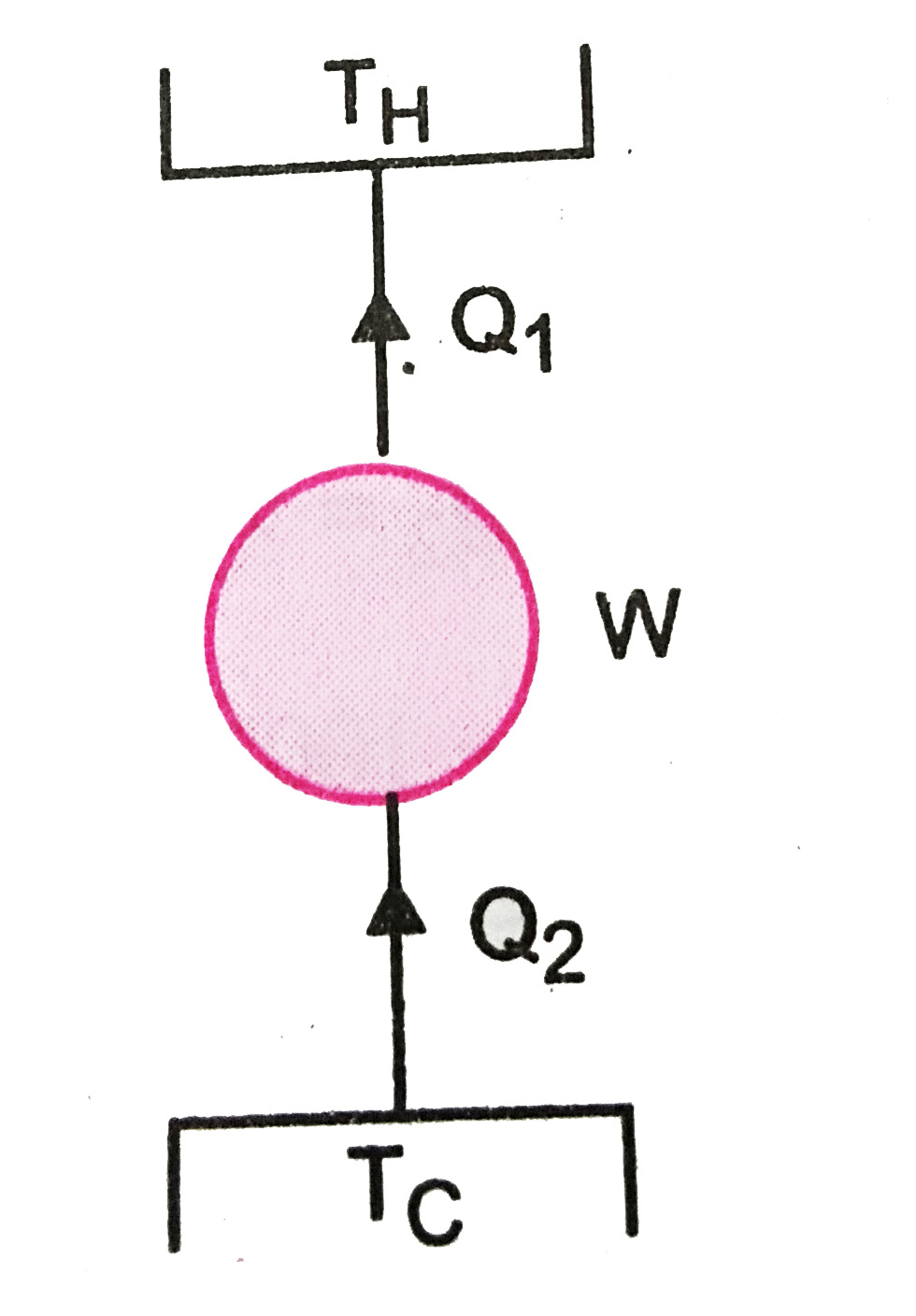
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THERMODYNAMICS
PRADEEP|Exercise Interger Type questions|11 VideosTHERMODYNAMICS
PRADEEP|Exercise Assertion- Reason Type Questions|19 VideosTHERMODYNAMICS
PRADEEP|Exercise Multiple choice questions (NCERT)|10 VideosSYSTEMS OF PARTICLES AND ROTATIONAL MOTION
PRADEEP|Exercise Assertion- Reason Type questions|20 VideosWORK, ENERGY AND POWER
PRADEEP|Exercise Assertion-Reason Type Questions|24 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-THERMODYNAMICS-Multiple choice questions.
- A Carnot engine, having an efficiency of eta=1//10 as heat engine, is ...
Text Solution
|
- Certain quantity of water cools from 70^(@)C to 60^(@)C in the first 5...
Text Solution
|
- The cofficient of performance of a refrigerator is 5. If the temperatu...
Text Solution
|
- A refrigerator works between 4^(@)C and 30^(@)C. It is required to rem...
Text Solution
|
- A carnot engine working between 300 K and 600 K has work output of 800...
Text Solution
|
- A Carnot engine takes 3xx10^6cal. of heat from a reservoir at 62^@C, a...
Text Solution
|
- The door of a running refrigerator inside a room is left open. The cor...
Text Solution
|
- A cannot engine has efficiency (1)/(6). If temperature of sink is decr...
Text Solution
|
- An ideal gas heat engine operates in Carnot cycle between 227^(@)C and...
Text Solution
|
- A Carnot engine whose sinl is at 300K has an efficiency of 40%. By how...
Text Solution
|
- A cannot engine has efficiency (1)/(6). If temperature of sink is decr...
Text Solution
|
- A Carnot engine, having an efficiency of eta=1//10 as heat engine, is ...
Text Solution
|
- A refrigerator with COP= 1//3 release 200 J at heat to a reservoir. Th...
Text Solution
|
- A Carnot engine operating between temperature T1 and T2 has efficiency...
Text Solution
|
- An ideal gas heat engine operates in a Carnot cycle between 227^(@)C a...
Text Solution
|
- A Carnot engine, whose efficiency is 40%, takes in heat from a source ...
Text Solution
|
- The above p-v diagram represents the thermodynamic cycle of an engine,...
Text Solution
|
- If a piece of metal is heated to temperature theta and the allowed to ...
Text Solution
|
- The work done in which of the following processes is zero
Text Solution
|
- In which of the following process (es), there is no change in the inte...
Text Solution
|