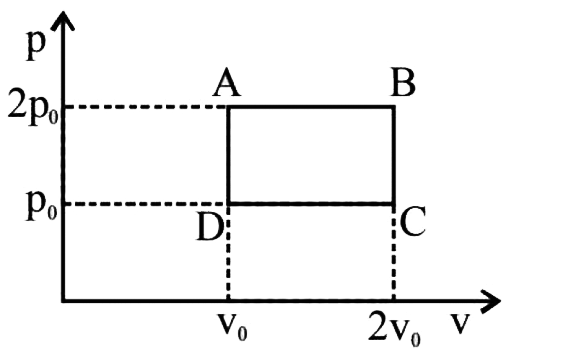
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THERMODYNAMICS
PRADEEP|Exercise Interger Type questions|11 VideosTHERMODYNAMICS
PRADEEP|Exercise Assertion- Reason Type Questions|19 VideosTHERMODYNAMICS
PRADEEP|Exercise Multiple choice questions (NCERT)|10 VideosSYSTEMS OF PARTICLES AND ROTATIONAL MOTION
PRADEEP|Exercise Assertion- Reason Type questions|20 VideosWORK, ENERGY AND POWER
PRADEEP|Exercise Assertion-Reason Type Questions|24 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-THERMODYNAMICS-Multiple choice questions.
- An ideal gas heat engine operates in a Carnot cycle between 227^(@)C a...
Text Solution
|
- A Carnot engine, whose efficiency is 40%, takes in heat from a source ...
Text Solution
|
- The above p-v diagram represents the thermodynamic cycle of an engine,...
Text Solution
|
- If a piece of metal is heated to temperature theta and the allowed to ...
Text Solution
|
- The work done in which of the following processes is zero
Text Solution
|
- In which of the following process (es), there is no change in the inte...
Text Solution
|
- In a process on a system, the initial pressure and volume are equal to...
Text Solution
|
- The pressure p and volume V of an ideal gas both increase in a process...
Text Solution
|
- Let Q and W denote the amount of heat given to an ideal gas and the wo...
Text Solution
|
- A gas kept in a container of finite conductivity is suddenly compresse...
Text Solution
|
- Refer to figure DeltaU(1) and DeltaU(2) be the changes in internal ene...
Text Solution
|
- One mole of an ideal gas in initial state A undergoes a cyclic process...
Text Solution
|
- A system goes from A and B via two processes. I and II as shown in fig...
Text Solution
|
- The temperature -entropy diagram of a reversible engine cycle is given...
Text Solution
|
- The internal enegy of an ideal gas decreases by the same amount as the...
Text Solution
|
- Which of the following graphs correctly represents the variation of be...
Text Solution
|
- A gas is copmressed isothermally to half its volume. BY what factor do...
Text Solution
|
- A gas is compressed adiabatically to half its volume. By what factor d...
Text Solution
|
- A gas is suddenly compressed to 1/4th of its original volume. Caculate...
Text Solution
|
- A Carnot engine absorbs 6xx10^(5)cal. At 227^(@)C. Heat rejected to th...
Text Solution
|