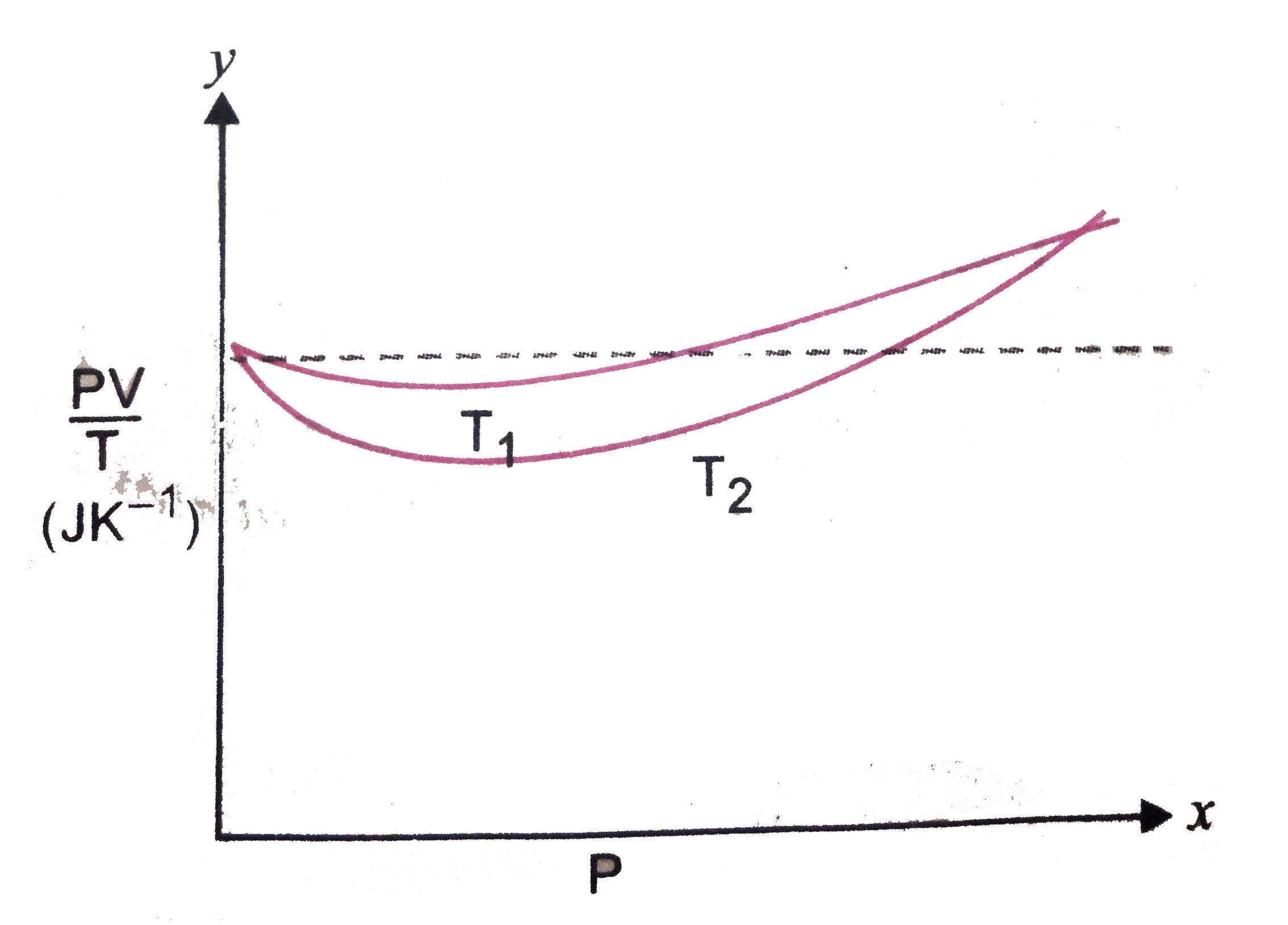Text Solution
Verified by Experts
Topper's Solved these Questions
BEHAVIOUR OF PERFECT GAS & KINETIC THEORY
PRADEEP|Exercise Higher order thinking skills questions|9 VideosBEHAVIOUR OF PERFECT GAS & KINETIC THEORY
PRADEEP|Exercise Value Based Questions|15 VideosBEHAVIOUR OF PERFECT GAS & KINETIC THEORY
PRADEEP|Exercise Advance problem for competitions|10 VideosGRAVIATION
PRADEEP|Exercise Assertion-Reason Type Questions|19 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-BEHAVIOUR OF PERFECT GAS & KINETIC THEORY-NCERT questions
- Estimate the fraction of molecular volume to the actual volume occupie...
Text Solution
|
- Molar volume is the volume occupied by 1 mole of any (Ideal) gas at st...
Text Solution
|
- Fig shows of PV//T versus P for 1.00 xx 10^(-3) kg of oxygen gas at tw...
Text Solution
|
- An oxygen cylinder of volume 30 litres has an initial gauge pressure o...
Text Solution
|
- An air bubble of volume 1.0 cm^(3) rises from the bottom of a take 40 ...
Text Solution
|
- Estimate the total number of air molecules (inclusive of oxygen, nitro...
Text Solution
|
- Estimate the average thermal energy of a helium atom at (i) room tempe...
Text Solution
|
- Three vessel of equal capacity have gases at the same temperature and ...
Text Solution
|
- At what temperature is the root mean square speed of an atom in an arg...
Text Solution
|
- Estimate the mean free path and collision frequency of a nitrogen mole...
Text Solution
|
- A metre long narrow bore held horizontally (and close at one end) cont...
Text Solution
|
- From a certain apparatus, the diffusion rate of hydrogen has an averag...
Text Solution
|
- A gas in equilibrium has uniform density and pressure throughout its v...
Text Solution
|