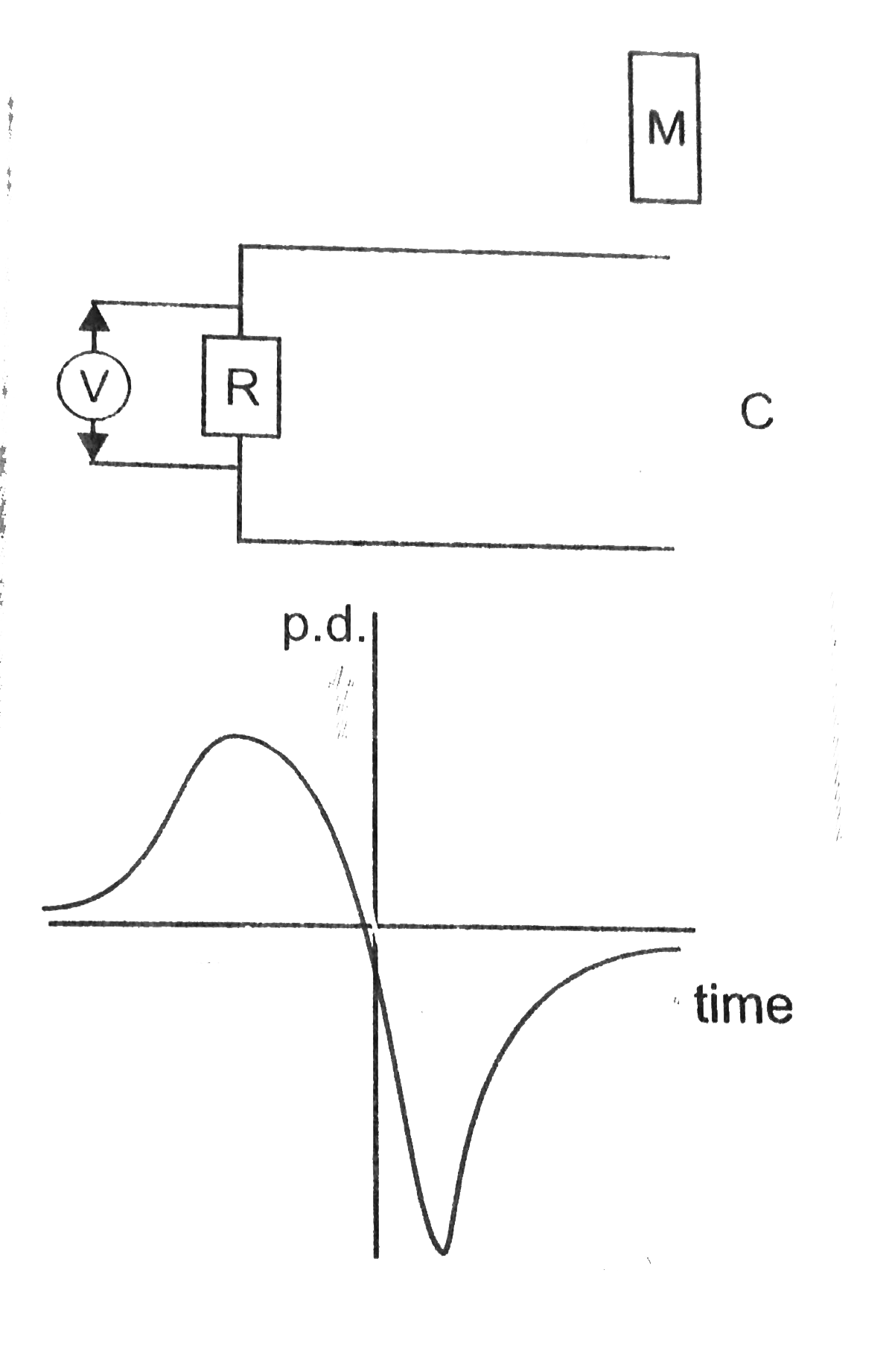
A bar magnet M is dropped so that if falls vertically through the coil C. The graph obtained for voltage produced acorss the coil vs time is shown in Fig.
(i) Explain the shape of the graph
(ii) Why is the negative peak longer than the positive peak?
A bar magnet M is dropped so that if falls vertically through the coil C. The graph obtained for voltage produced acorss the coil vs time is shown in Fig.
(i) Explain the shape of the graph
(ii) Why is the negative peak longer than the positive peak?

(i) Explain the shape of the graph
(ii) Why is the negative peak longer than the positive peak?

Text Solution
Verified by Experts
As the magnet M approaches coil C, magnetic flux linked with the coil increases. `emf//current` is induced in the coil, which opposes the increase in flux. When magnet in inside the coil, magnetic flux linked with the coil is constant. Induced `emf//current` is zero. As the magnet falls below the coil, mag. flux linked with the coil decreases. `emf//current` is induced in the coil, which opposes the decrease in flux. As velocity of magent has increased, induced `emf//current` is more. Therefore, nagative peak is longer than the positive peak. When the magnet has fallen through large distance, changing mag. flux due to its movement vanishes. Induced emf reduces to zero.
Topper's Solved these Questions
ELECTROMAGNETIC INDUCTION & ALTERNATING CURRENT
PRADEEP|Exercise Solved Examples (b)|1 VideosELECTROMAGNETIC INDUCTION & ALTERNATING CURRENT
PRADEEP|Exercise Short Answer Qusetions|2 VideosDUAL NATURE OF RADIATION AND MATTER
PRADEEP|Exercise Exercise|191 VideosELECTROMAGNETIC WAVES
PRADEEP|Exercise II Focus multiple choice question|5 Videos
Similar Questions
Explore conceptually related problems
The current through the coil in figure (i) varies as shown in figure (ii). Which graph best shows the ammeter A reading as a function of time?
(a) Draw graph showing the variation of current versus voltage in an electroyte when an external resistance is also connceted. (b) (i) The graph between resistance (R ) and temperature (T) for Hg is shown in the figure (a). Explain the behaviour of Hg near 4k. (ii) In which region of the graph shown in the figure (b) is the resistance negative and why?
Consider a box with three terminals on top of it as shown in figure. Three components namely, two germanium diodes and one resistor are connected across these three terminals in some arrangement A student performs an experiment in which any two of these three terminals are connected in the circuit shown in figure. The student obtains graphs of current-voltage characteristics for unknown combination of components between the two terminals connected in the circuit. The graphs are (i) When A is positive and B is negative (ii) When A is negative and B is positive (iii) When B is negative and C is positve (iv) When B is positive and C is neagtive (v) When A is positive and C is negative (vi) When A is negative and C is positive From these graphs of current - voltage characteristic shown in fig. (c) to (h) determine the arrangement of components between A, B and C.
The existence of negatively charged particle in an atom was shown by J.J. Thomson as a result of the studies of the passage of electricity through gases at extremely low pressure known as discharge tube experiments. When a high voltage of the order of 10,000 volts or more was impressed across the electrodes, some sort of invisible rays moved from the negative electrode to the positive electrode these rays are called as cathode rays. Cathode rays travel in straight path in absence of electrical and magnetic field . Cathode rays consist of material part and charged particles? Cathode rays produce X-rays and light is emitted when they strike on ZnS screen. Cathode rays penetrate through thin sheets of aluminium and other metals . They affect the photogenic plate and passes heating effect when they strike on metal foil. The raito of charge to mass i.e charge/mass is same for all the cathode rays irrespective of the gas used in the tube. The existence of positively charged particle in an atom was shown be E. Goldstein. He repeated the same discharge tube experiments by using a perforated cathode. It was observed that when a high potential difference was applied between the electrodes, not only cathode rays were produced but also a new type of rays were produced simultaneoulsy from anode moving towards cathode and passes through the holes or canal of the cathode. These termed as canal rays or anode rays. These rays travel in straight lines and consists of positively charged particles. These rays have kinetic energy and produces heating effect also. The e/m ratio of these rays is smaller than that of electrons. Unlike cathode rays, their e/m value is dependent upon the nature of the gas taken in the tube. These rays produced flashes of light on ZnS screen and can pass throughs thin metal foils. They can produce physical and chemical changes and are capable to produce ionisation in gases. For cathode rays the value of e/m:
The existence of negatively charged particle in an atom was shown by J.J. Thomson as a result of the studies of the passage of electricity through gases at extremely low pressure known as discharge tube experiments. When a high voltage of the order of 10,000 volts or more was impressed across the electrodes, some sort of invisible rays moved from the negative electrode to the positive electrode these rays are called as cathode rays. Cathode rays travel in straight path in absence of electrical and magnetic field . Cathode rays consist of material part and charged particles? Cathode rays produce X-rays and light is emitted when they strike on ZnS screen. Cathode rays penetrate through thin sheets of aluminium and other metals . They affect the photogenic plate and passes heating effect when they strike on metal foil. The raito of charge to mass i.e charge/mass is same for all the cathode rays irrespective of the gas used in the tube. The existence of positively charged particle in an atom was shown be E. Goldstein. He repeated the same discharge tube experiments by using a perforated cathode. It was observed that when a high potential difference was applied between the electrodes, not only cathode rays were produced but also a new type of rays were produced simultaneoulsy from anode moving towards cathode and passes through the holes or canal of the cathode. These termed as canal rays or anode rays. These rays travel in straight lines and consists of positively charged particles. These rays have kinetic energy and produces heating effect also. The e/m ratio of these rays is smaller than that of electrons. Unlike cathode rays, their e/m value is dependent upon the nature of the gas taken in the tube. These rays produced flashes of light on ZnS screen and can pass throughs thin metal foils. They can produce physical and chemical changes and are capable to produce ionisation in gases. Select the incorrect statement.
The existence of negatively charged particle in an atom was shown by J.J. Thomson as a result of the studies of the passage of electricity through gases at extremely low pressure known as discharge tube experiments. When a high voltage of the order of 10,000 volts or more was impressed across the electrodes, some sort of invisible rays moved from the negative electrode to the positive electrode these rays are called as cathode rays. Cathode rays travel in straight path in absence of electrical and magnetic field . Cathode rays consist of material part and charged particles? Cathode rays produce X-rays and light is emitted when they strike on ZnS screen. Cathode rays penetrate through thin sheets of aluminium and other metals . They affect the photogenic plate and passes heating effect when they strike on metal foil. The raito of charge to mass i.e charge/mass is same for all the cathode rays irrespective of the gas used in the tube. The existence of positively charged particle in an atom was shown be E. Goldstein. He repeated the same discharge tube experiments by using a perforated cathode. It was observed that when a high potential difference was applied between the electrodes, not only cathode rays were produced but also a new type of rays were produced simultaneoulsy from anode moving towards cathode and passes through the holes or canal of the cathode. These termed as canal rays or anode rays. These rays travel in straight lines and consists of positively charged particles. These rays have kinetic energy and produces heating effect also. The e/m ratio of these rays is smaller than that of electrons. Unlike cathode rays, their e/m value is dependent upon the nature of the gas taken in the tube. These rays produced flashes of light on ZnS screen and can pass throughs thin metal foils. They can produce physical and chemical changes and are capable to produce ionisation in gases. Which is not true with respect to cathode rays?
A vertical bar magnet is dropped from from position on the axis of a fixed metallic coil as shown in fig-I. In fig - II the magnet is fixed and horizontal coil is dropped. The accelaration of the magnet and coil are a_(1) and a_(2) respectively then