Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
PRADEEP-DUAL NATURE OF RADIATION AND MATTER-Exercise
- The main aim of Davisson-Germer experiment is to verify............ .
Text Solution
|
- Given in fig. is the graph between frequency v of the incident light a...
Text Solution
|
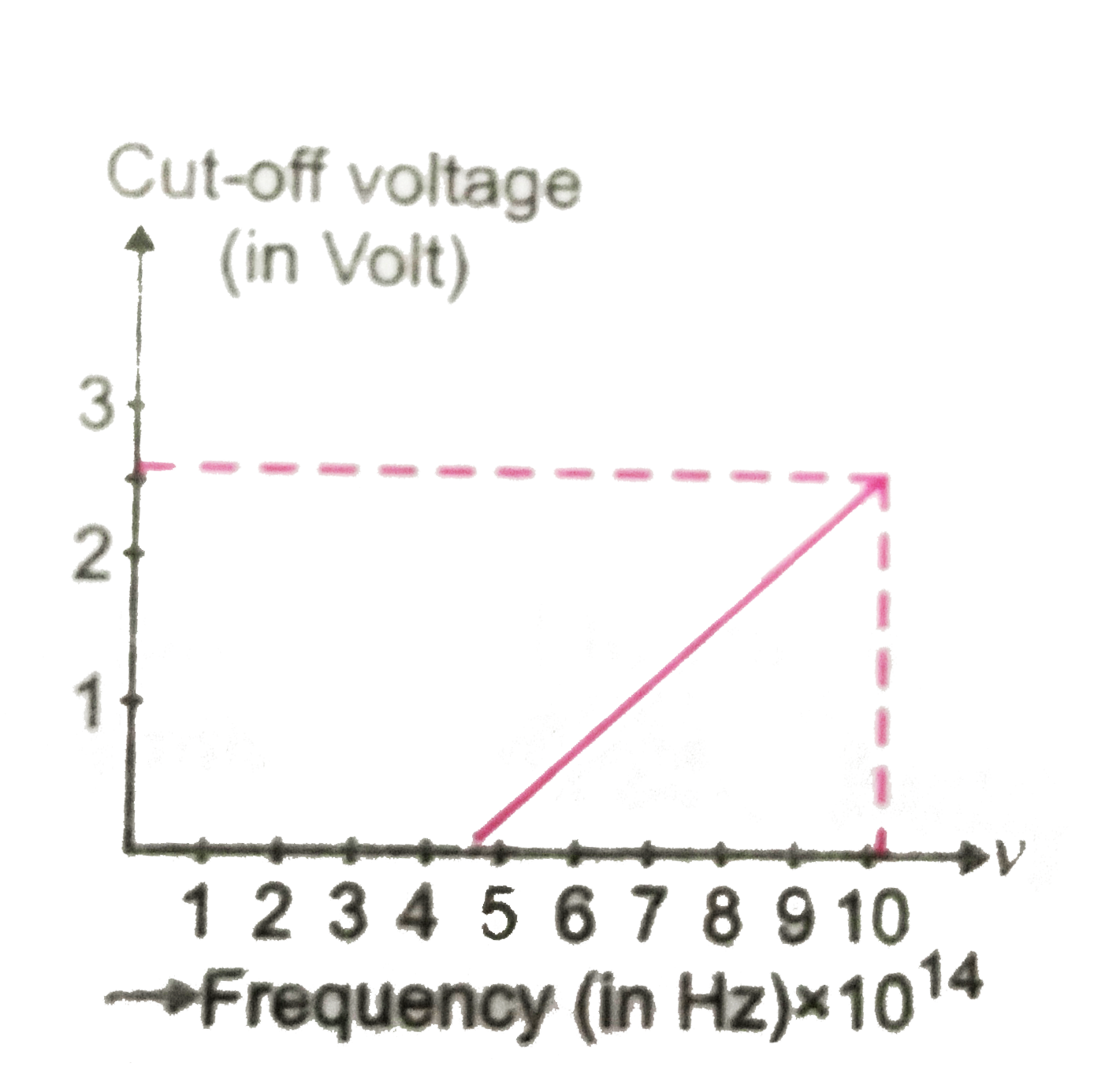
- For photoelectric effect in sodium, fig. shows the plot of cut-off vol...
Text Solution
|
- When light of wavelength 400nm is incident on the cathode of photocell...
Text Solution
|
- Radiation of a certain wavelength causes electrons with a maximum kine...
Text Solution
|
- The electric field associated with a monochromataic beam of light beco...
Text Solution
|
- Ultraviolet light of wavelength 800 A and 700 A when allowed to fall ...
Text Solution
|
- Light of wavelength 2000 Å falls on an aluminium surface . In aluminiu...
Text Solution
|
- The maximum wavelength for which an em wave can ejected electrons form...
Text Solution
|
- Find the frequency of light which ejects electrons from a metal surfac...
Text Solution
|
- The work fuction of caseium is 1.8 eV. Light of 4500Å is incident on i...
Text Solution
|
- Find the difference of kinetic energies of photoelectrons emitted from...
Text Solution
|
- The electric field associated with a light wave is given by E=E(0)sin[...
Text Solution
|
- A photon of wavelength 3310Å falls on a photocathode and an electron o...
Text Solution
|
- Light of wavelength 180 nm ejects photoelectrons from a plate of met...
Text Solution
|
- The maximum velcities of the photoelectrons ejected are v and 2v for i...
Text Solution
|
- A radio transmitter operates at a frequency of 880 kHz and a power of ...
Text Solution
|
- The minimum intensity of light to be detected by human eye is 10^(-10)...
Text Solution
|
- Find the number of photons emitted per minute by a 25W source of monoc...
Text Solution
|
- Monochromatic light of frequency 5.0xx10^(14)Hz is produced by a laser...
Text Solution
|