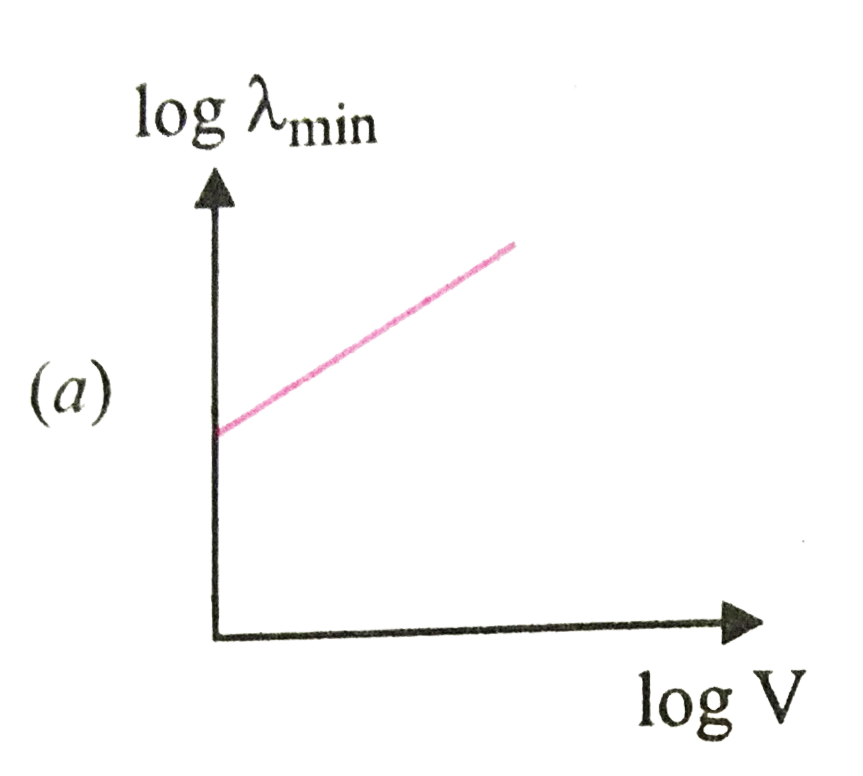
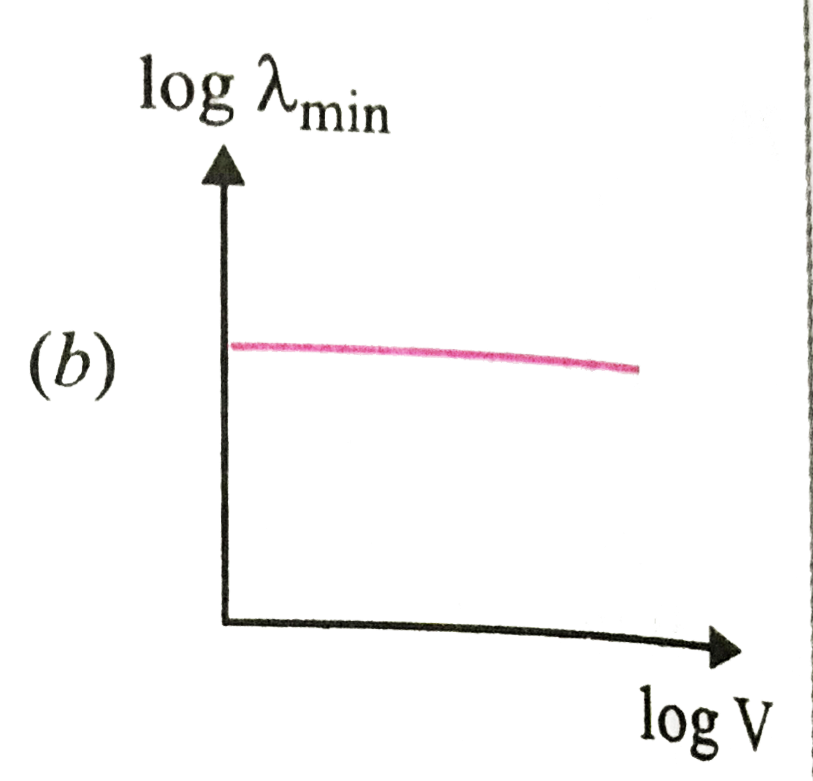
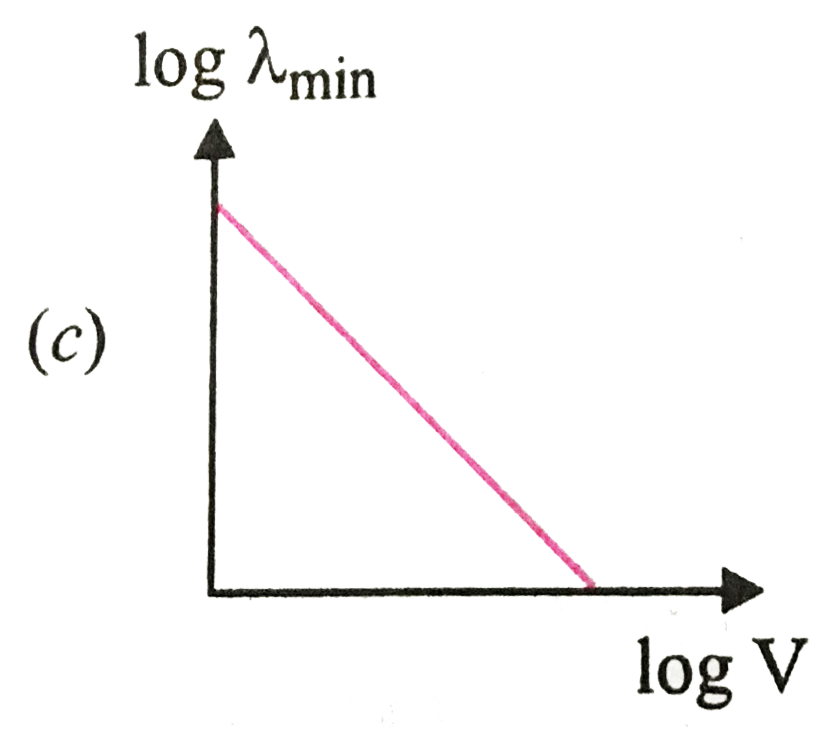
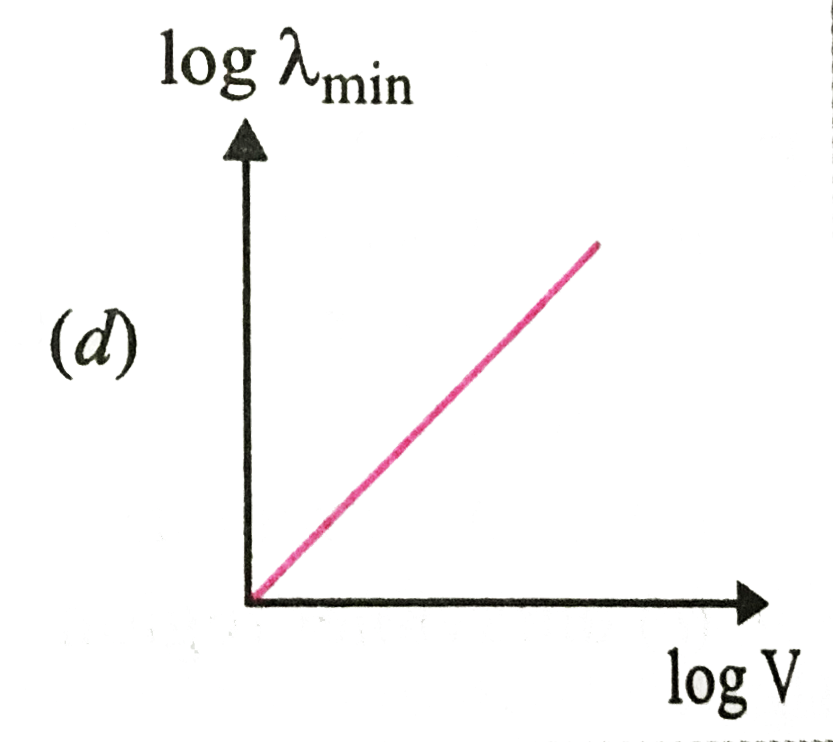
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
PRADEEP-DUAL NATURE OF RADIATION AND MATTER-Exercise
- A parallel beam of the light is incident normally on a plane surface a...
Text Solution
|
- A beam of light of wavelength 400nm and power 1.55 mW is directed at t...
Text Solution
|
- An electron beam is acceleration by a potential difference V to hit a ...
Text Solution
|
- Which of the following figure represents the variation of particle mom...
Text Solution
|
- An X-ray tube operates at 10kV. Find the ratio of X-rays wavelength to...
Text Solution
|
- The de - Broglie wavelength of a neutron in thermal equilibrium with h...
Text Solution
|
- If lambda(1) and lambda(2) denote the wavelength of de-broglie waves f...
Text Solution
|
- The energy that should be added to an electron, to reduce its de-Brogl...
Text Solution
|
- The ratio between masses of two particles is 1:2 and ratio between the...
Text Solution
|
- For Bragg's differaction by a crystal to occur, then the X-ray of wave...
Text Solution
|
- The de-Broglie wavelength of the tennis ball of mass 60g moving with a...
Text Solution
|
- The de-Broglie wavelength associated with proton changes by 0.25% if i...
Text Solution
|
- The de - Broglie wavelength of a particle moving with a velocity 2.25 ...
Text Solution
|
- The energy of a photon is equal to the kinetic energy of a proton. T...
Text Solution
|
- If the kinetic energy of the particle is increased to 16 times its pre...
Text Solution
|
- The ratio of de - Broglie wavelength of alpha - particle to that of a ...
Text Solution
|
- Find the ratio of de Broglie wavelength of molecules of hydrogen and h...
Text Solution
|
- What is the de - Broglie wavelength of the alpha - particle accelerate...
Text Solution
|
- A particle A of mass m and initial velocity v collides with a particle...
Text Solution
|
- A free particle with initial kinetic energy E, de-Broglie wavelength l...
Text Solution
|